Dihydrocholesterol
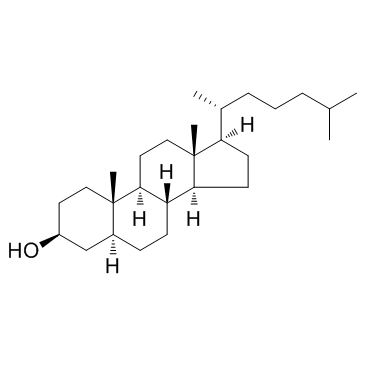
Dihydrocholesterol structure
|
Common Name | Dihydrocholesterol | ||
|---|---|---|---|---|
| CAS Number | 80-97-7 | Molecular Weight | 388.669 | |
| Density | 1.0±0.1 g/cm3 | Boiling Point | 455.5±13.0 °C at 760 mmHg | |
| Molecular Formula | C27H48O | Melting Point | 140-142ºC | |
| MSDS | USA | Flash Point | 190.7±12.3 °C | |
| Symbol |


GHS06, GHS08 |
Signal Word | Danger | |
Use of Dihydrocholesterol5α-Cholestan-3β-ol is a derivitized steroid compound, which is isolated from the testes of White Carneau pigeons. |
| Name | (5α)-cholestan-3β-ol |
|---|---|
| Synonym | More Synonyms |
| Description | 5α-Cholestan-3β-ol is a derivitized steroid compound, which is isolated from the testes of White Carneau pigeons. |
|---|---|
| Related Catalog | |
| Target |
Human Endogenous Metabolite |
| In Vitro | 5α-Cholestan-3β-ol is derived from cholesterol by the action of intestinal microorganisms. It is known to induce the formation of gall stones in rabbits in the presence of sodium ions. 5α-Cholestan-3β-ol is used as a standard in lipid analysis using HPLC. |
| References |
| Density | 1.0±0.1 g/cm3 |
|---|---|
| Boiling Point | 455.5±13.0 °C at 760 mmHg |
| Melting Point | 140-142ºC |
| Molecular Formula | C27H48O |
| Molecular Weight | 388.669 |
| Flash Point | 190.7±12.3 °C |
| Exact Mass | 388.370514 |
| PSA | 20.23000 |
| LogP | 10.22 |
| Vapour Pressure | 0.0±2.5 mmHg at 25°C |
| Index of Refraction | 1.504 |
| Storage condition | room temp |
| Stability | Stable. Combustible. Incompatible with strong oxidizing agents. |
| Water Solubility | chloroform: 0.1 g/mL, clear, colorless |
| Symbol |


GHS06, GHS08 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H302-H315-H319-H331-H336-H351-H361d-H372 |
| Precautionary Statements | P261-P281-P305 + P351 + P338-P311 |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
| Hazard Codes | Xn: Harmful; |
| Risk Phrases | R22 |
| Safety Phrases | S22-S24/25 |
| RIDADR | UN 1888 6 |
| WGK Germany | 3 |
| RTECS | FZ6350000 |
| Precursor 8 | |
|---|---|
| DownStream 9 | |
|
Synthesis of steryl ferulates with various sterol structures and comparison of their antioxidant activity.
Food Chem. 169 , 92-101, (2014) Steryl ferulates synthesised from commercial sterols as well as commercial oryzanol were used to better understand how structural features affect antioxidant activity in vitro by the ABTS(+) radical d... |
|
|
Novel and efficient synthesis and antifungal evaluation of 2,3-functionalized cholestane and androstane derivatives
Bioorg. Med. Chem. Lett. 20 , 7372-5, (2010) Synthetic modifications of cholesterol and other traditional steroid molecules have become a promising area for the exploration and development of novel antifungal agents, especially with respect to t... |
|
|
An integrated evaluation of molecular marker indices and linear alkylbenzenes (LABs) to measure sewage input in a subtropical estuary (Babitonga Bay, Brazil).
Environ. Pollut. 188 , 71-80, (2014) Babitonga Bay is a South Atlantic estuary with significant ecological function; it is part of the last remaining areas of mangrove communities in the Southern Hemisphere. The aim of this study was to ... |
| b-Cholestanol |
| 5a-Cholestan-3b-ol |
| (5α)-cholestan-3β-ol |
| 5a-Dihydrocholesterol |
| 3.β.-Hydroxy-5.α.-cholestane |
| (5alpha)-cholestan-3beta-ol |
| (3S,5S,8R,9S,10S,13R,14S,17R)-10,13-Dimethyl-17-[(2R)-6-methyl-2-heptanyl]hexadecahydro-1H-cyclopenta[a]phenanthren-3-ol |
| 5α-Cholestan-3β-ol |
| (3β,5α)-Cholestan-3-ol |
| β-Cholestanol |
| 3b-Hydroxy-5a-cholestane |
| 3β-Hydroxy-5α-cholestane |
| Cholestan-3-ol, (3β,5α)- |
| 5α-Dihydrocholesterol |
| Cholestan-3-ol |
| cholestanol |
| EINECS 201-315-8 |
| MFCD00066413 |
| (3b,5a)-Cholestan-3-ol |
| Cholestanol (VAN) |
| (3S,5S,8R,9S,10S,13R,14S,17R)-10,13-Diméthyl-17-[(2R)-6-méthyl-2-heptanyl]hexadécahydro-1H-cyclopenta[a]phénanthrén-3-ol |
| beta-cholrestanol |
 CAS#:601-57-0
CAS#:601-57-0 CAS#:57-88-5
CAS#:57-88-5 CAS#:1255-88-5
CAS#:1255-88-5 CAS#:566-88-1
CAS#:566-88-1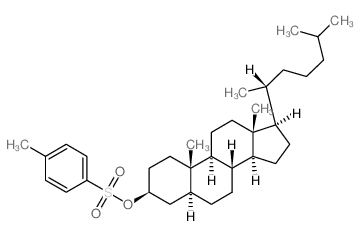 CAS#:3381-52-0
CAS#:3381-52-0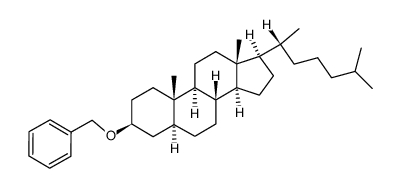 CAS#:69483-57-4
CAS#:69483-57-4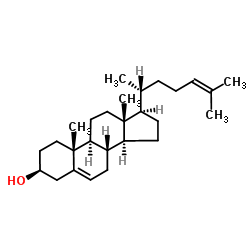 CAS#:313-04-2
CAS#:313-04-2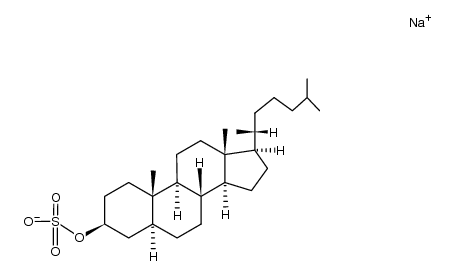 CAS#:107584-99-6
CAS#:107584-99-6![Cyclopenta[5,6]naphth[1,2-d]azepin-2 (1H)-one, 8.beta.-(1, 5-dimethylhexyl)-3,4,5,5a,5b.alpha.,6,7,7a,8,9,10,10a.alpha., 10b.beta.,11,12,12a.alpha.-hexadecahydro-5a.beta., 7a.beta.-dimethyl- structure](https://image.chemsrc.com/caspic/407/20283-99-2.png) CAS#:20283-99-2
CAS#:20283-99-2 CAS#:481-21-0
CAS#:481-21-0 CAS#:10437-24-8
CAS#:10437-24-8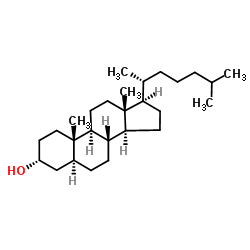 CAS#:516-95-0
CAS#:516-95-0 CAS#:1178-00-3
CAS#:1178-00-3 CAS#:571-31-3
CAS#:571-31-3![(3S,5S,8R,9S,10S,13R,14S,17R)-10,13-dimethyl-17-[(2R)-6-methylheptan-2-yl]-3-methylsulfonyloxy-2,3,4,5,6,7,8,9,11,12,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthrene structure](https://image.chemsrc.com/caspic/160/3381-51-9.png) CAS#:3381-51-9
CAS#:3381-51-9 CAS#:3381-54-2
CAS#:3381-54-2
