cholesterol
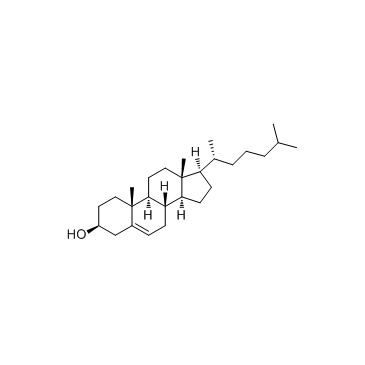
cholesterol structure
|
Common Name | cholesterol | ||
|---|---|---|---|---|
| CAS Number | 57-88-5 | Molecular Weight | 386.654 | |
| Density | 1.0±0.1 g/cm3 | Boiling Point | 360 ºC | |
| Molecular Formula | C27H46O | Melting Point | 148-150 °C | |
| MSDS | Chinese USA | Flash Point | 250 ºC | |
| Symbol |


GHS06, GHS08 |
Signal Word | Danger | |
Use of cholesterolCholesterol is the major sterol in mammals, and its importance in fundamental cellular processes is becoming more appreciated. IC50 value:Target:In vitro: GT1-7 hypothalamic cells subjected to cholesterol depletion in vitro produced 20-31% reductions in cellular cholesterol content, similar to the decrease in cholesterol synthesis observed in diabetes [1].In vivo: |
| Name | cholesterol |
|---|---|
| Synonym | More Synonyms |
| Description | Cholesterol is the major sterol in mammals, and its importance in fundamental cellular processes is becoming more appreciated. IC50 value:Target:In vitro: GT1-7 hypothalamic cells subjected to cholesterol depletion in vitro produced 20-31% reductions in cellular cholesterol content, similar to the decrease in cholesterol synthesis observed in diabetes [1].In vivo: |
|---|---|
| Related Catalog | |
| Target |
Human Endogenous Metabolite |
| References |
| Density | 1.0±0.1 g/cm3 |
|---|---|
| Boiling Point | 360 ºC |
| Melting Point | 148-150 °C |
| Molecular Formula | C27H46O |
| Molecular Weight | 386.654 |
| Flash Point | 250 ºC |
| Exact Mass | 386.354858 |
| PSA | 20.23000 |
| LogP | 9.85 |
| Vapour Pressure | 0.0±2.7 mmHg at 25°C |
| Index of Refraction | 1.525 |
| Water Solubility | negligible |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Symbol |


GHS06, GHS08 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H302-H315-H319-H331-H336-H351-H361d-H372 |
| Precautionary Statements | P201-P261-P304 + P340 + P312-P305 + P351 + P338-P308 + P313-P403 + P233 |
| Personal Protective Equipment | Eyeshields;Faceshields;full-face respirator (US);Gloves;multi-purpose combination respirator cartridge (US) |
| Hazard Codes | Xn |
| Risk Phrases | R10 |
| Safety Phrases | S24/25-S22-S36/37 |
| RIDADR | UN 1170 3/PG 3 |
| WGK Germany | 1 |
| RTECS | FZ8400000 |
| Hazard Class | 3.0 |
| HS Code | 2906131000 |
| Precursor 10 | |
|---|---|
| DownStream 10 | |
| HS Code | 2906131000 |
|---|
|
Rehmannia glutinosa (Gaertn.) DC. polysaccharide ameliorates hyperglycemia, hyperlipemia and vascular inflammation in streptozotocin-induced diabetic mice.
J. Ethnopharmacol. 164 , 229-38, (2015) Rehmannia glutinosa (Gaertn.) DC. (RG) has been widely used as traditional Chinese herbal medicine for treatment of diabetes and its complications. The polysaccharide fraction of RG has been proposed ... |
|
|
G-protein-coupled estrogen receptor 1 is anatomically positioned to modulate synaptic plasticity in the mouse hippocampus.
J. Neurosci. 35(6) , 2384-97, (2015) Both estrous cycle and sex affect the numbers and types of neuronal and glial profiles containing the classical estrogen receptors α and β, and synaptic levels in the rodent dorsal hippocampus. Here, ... |
|
|
Activation of Tomato Bushy Stunt Virus RNA-Dependent RNA Polymerase by Cellular Heat Shock Protein 70 Is Enhanced by Phospholipids In Vitro.
J. Virol. 89(10) , 5714-23, (2015) Similar to other positive-strand RNA viruses, tombusviruses are replicated by the membrane-bound viral replicase complex (VRC). The VRC consists of the p92 virus-coded RNA-dependent RNA polymerase (Rd... |
| 20-Dihydro-11-deoxycortisol |
| cholesterin |
| D5-Cholesten-3b-ol |
| 20-Dihydro Cortexolone |
| 3b-Hydroxy-5-cholestene |
| 5:6-Cholesten-3b-ol |
| (3β)-Cholest-5-en-3-ol |
| δ5-Cholesten-3β-ol |
| cholest-5-en-3-ol, (3b)- |
| Cholest-5-en-3-ol (3β)- |
| Cholest-5-en-3b-ol |
| EINECS 200-353-2 |
| 5-Cholesten-3B-ol |
| 5-Cholesten-3β-ol |
| Cholesterol |
| (3b)-cholest-5-en-3-ol |
| 3β-Hydroxycholest-5-ene |
| 5:6-Cholesten-3β-ol |
| (3S,8S,9S,10R,13R,14S,17R)-10,13-Dimethyl-17-[(2R)-6-methyl-2-heptanyl]-2,3,4,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthren-3-ol |
| 7α-Cholesterol |
| MFCD00003646 |
| Cholest-5-en-3-ol, (3β)- |
| (-)-Cholesterol |
| Cholest-5-en-3β-ol |
 CAS#:57711-50-9
CAS#:57711-50-9 CAS#:33999-75-6
CAS#:33999-75-6 CAS#:7604-85-5
CAS#:7604-85-5 CAS#:25092-65-3
CAS#:25092-65-3![3β-[(Tetrahydro-2H-pyran)-2-yloxy]cholest-5-ene Structure](https://image.chemsrc.com/caspic/185/6252-45-5.png) CAS#:6252-45-5
CAS#:6252-45-5![17-(1,5-dimethyl-hexyl)-10,13-dimethyl-2,3,4,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-1Hcyclopenta[a]phenanthren-3-yl diphenyl phosphate Structure](https://image.chemsrc.com/caspic/151/24352-63-4.png) CAS#:24352-63-4
CAS#:24352-63-4![(2,2,7,7-Tetramethyltetrahydro-3aH-bis[1,3]dioxolo[4,5-b:4',5'-d]pyran-5-yl)methanol Structure](https://image.chemsrc.com/caspic/222/4064-06-6.png) CAS#:4064-06-6
CAS#:4064-06-6 CAS#:1857-80-3
CAS#:1857-80-3 CAS#:2867-93-8
CAS#:2867-93-8 CAS#:70650-16-7
CAS#:70650-16-7 CAS#:601-34-3
CAS#:601-34-3 CAS#:35602-69-8
CAS#:35602-69-8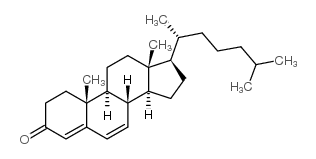 CAS#:566-93-8
CAS#:566-93-8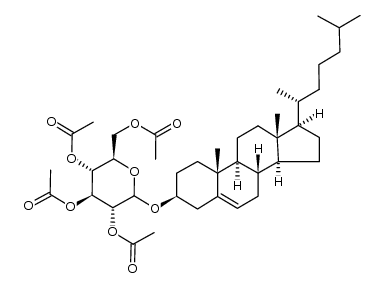 CAS#:1232680-81-7
CAS#:1232680-81-7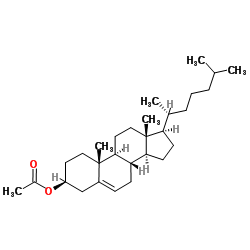 CAS#:604-35-3
CAS#:604-35-3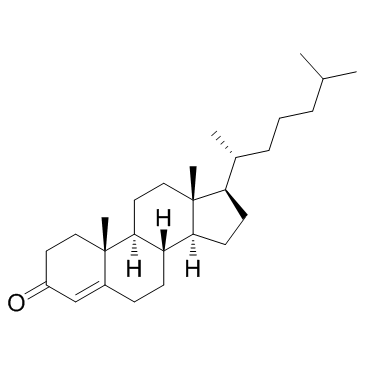 CAS#:601-57-0
CAS#:601-57-0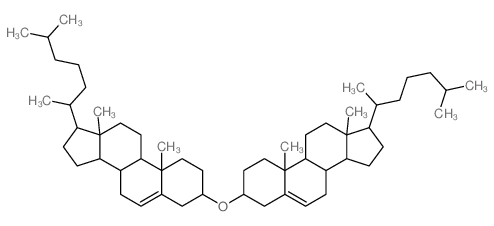 CAS#:2469-23-0
CAS#:2469-23-0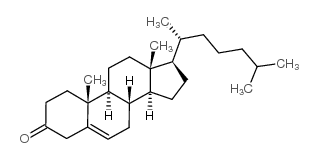 CAS#:601-54-7
CAS#:601-54-7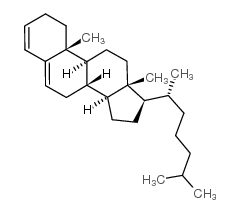 CAS#:747-90-0
CAS#:747-90-0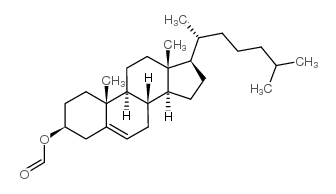 CAS#:4351-55-7
CAS#:4351-55-7
