Remoxipride

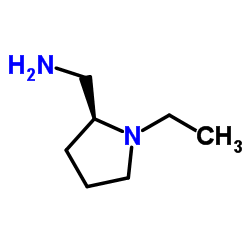
Remoxipride structure
|
Common Name | Remoxipride | ||
|---|---|---|---|---|
| CAS Number | 80125-14-0 | Molecular Weight | 371.26900 | |
| Density | 1.292 | Boiling Point | 439.9ºC at 760 mmHg | |
| Molecular Formula | C16H23BrN2O3 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | 219.8ºC | |
Use of Remoxipride(S)-Remoxipride ((-)-Remoxipride) is a selective dopamine D2-receptor antagonist with an IC50 value of 1.57 μM. (S)-Remoxipride can be used for the research of psychotic disorder[1]. |
| Name | 3-bromo-N-[[(2S)-1-ethylpyrrolidin-2-yl]methyl]-2,6-dimethoxybenzamide |
|---|---|
| Synonym | More Synonyms |
| Description | (S)-Remoxipride ((-)-Remoxipride) is a selective dopamine D2-receptor antagonist with an IC50 value of 1.57 μM. (S)-Remoxipride can be used for the research of psychotic disorder[1]. |
|---|---|
| Related Catalog | |
| Target |
D2 Receptor:1.57 μM (IC50) D1 Receptor:>100 μM (IC50) α1-Adrenoccptor:42 μM (IC50) |
| In Vitro | (S)-Remoxipride (1-100 μM; 20 min) shows binding efficiency with IC50s of >100, 1.57 and 42 μM for dopamine D1, dopamine D2 and α1-Adrenoccptor, respectively[1]. |
| In Vivo | (S)-Remoxipride (0.1-100 μM/kg; i.p. 60 min prior to apomorphine) blockades apomorphine-induced behaviors s in rats and vomiting in dogs[1]. (S)-Remoxipride (0.1-10 mg/kg; i.p. 30 min prior to apomorphine) displaces [3H]spiperone from both striatal and extra-striatal areas[1]. Animal Model: Male Sprague-Dawley rats[1] Dosage: 0.1-100 μM/kg Administration: Intraperitoneal injection; 0.1-100 μM/kg; 60 min prior to apomorphine Result: Blocked apomorphine-induced hyperactivity and dose-dependent blockaded apomorphine-induced behaviors in vivo. Animal Model: Male and female beagle dogs[1] Dosage: 0.25-5 μM/kg Administration: Oral gavage; 0.25-5 μM/kg; 60 min prior to apomorphine Result: Blocked apomorphine-induced vomiting in dogs. |
| Density | 1.292 |
|---|---|
| Boiling Point | 439.9ºC at 760 mmHg |
| Molecular Formula | C16H23BrN2O3 |
| Molecular Weight | 371.26900 |
| Flash Point | 219.8ºC |
| Exact Mass | 370.08900 |
| PSA | 50.80000 |
| LogP | 3.00920 |
| Index of Refraction | 1.54 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Hazard Codes | Xi |
|---|---|
| Risk Phrases | R36/37/38:Irritating to eyes, respiratory system and skin . |
| Safety Phrases | S26-S36-S24/25 |
| WGK Germany | 3 |
|
~% 
Remoxipride CAS#:80125-14-0 |
| Literature: Journal of Medicinal Chemistry, , vol. 25, # 11 p. 1280 - 1286 |
|
~% 
Remoxipride CAS#:80125-14-0 |
| Literature: Journal of Medicinal Chemistry, , vol. 25, # 11 p. 1280 - 1286 |
|
~% 
Remoxipride CAS#:80125-14-0 |
| Literature: Journal of Medicinal Chemistry, , vol. 25, # 11 p. 1280 - 1286 |
|
~% 
Remoxipride CAS#:80125-14-0 |
| Literature: Medicinal Chemistry Research, , vol. 6, # 2 p. 69 - 80 |
| Remoxipridum [INN-Latin] |
| (S)-remoxipride |
| (S)-3-bromo-N-[(1-ethylpyrrolidin-2-yl)methyl]-2,6-dimethoxybenzamide |
| Remoxiprida |
| (-)-N-ethyl-2-(3-bromo-2,6-dimethoxybenzamidomethyl)pyrrolidine |
| remoxipiride |
| S(-)-3-bromo-N-[(1-ethyl-2-pyrrolidinyl)methyl]-2,6-dimethoxy-benzamide |
| remoxipride |
| FLA-731 |
| Romoxipride |
| Roxiam |
| Remoxipride [USAN:BAN:INN] |
| Remoxiprida [INN-Spanish] |
| Remoxipridum |
| MFCD00869705 |