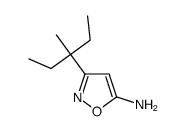
isoxaben
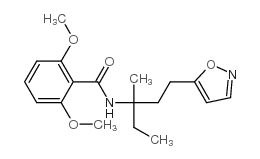
isoxaben structure
|
Common Name | isoxaben | ||
|---|---|---|---|---|
| CAS Number | 82558-50-7 | Molecular Weight | 332.39 | |
| Density | 1.138g/cm3 | Boiling Point | 427.8ºC at 760 mmHg | |
| Molecular Formula | C18H24N2O4 | Melting Point | 175-179ºC | |
| MSDS | Chinese USA | Flash Point | 212.6ºC | |
Use of isoxabenIsoxaben, a herbicide, inhibits incorporation of radiolabeled glucose into an acid insoluble cell wall fraction. Isoxaben is also a specific inhibitor of cell wall biosynthesis[1]. |
| Name | isoxaben |
|---|---|
| Synonym | More Synonyms |
| Description | Isoxaben, a herbicide, inhibits incorporation of radiolabeled glucose into an acid insoluble cell wall fraction. Isoxaben is also a specific inhibitor of cell wall biosynthesis[1]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.138g/cm3 |
|---|---|
| Boiling Point | 427.8ºC at 760 mmHg |
| Melting Point | 175-179ºC |
| Molecular Formula | C18H24N2O4 |
| Molecular Weight | 332.39 |
| Flash Point | 212.6ºC |
| Exact Mass | 332.17400 |
| PSA | 73.59000 |
| LogP | 3.61400 |
| Index of Refraction | 1.544 |
| Storage condition | 0-6°C |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Hazard Statements | H413 |
|---|---|
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xn: Harmful; |
| Risk Phrases | 53-40 |
| Safety Phrases | S36 |
| RIDADR | NONH for all modes of transport |
| RTECS | CV4370300 |
|
~91% 
isoxaben CAS#:82558-50-7 |
| Literature: Eli Lilly and Company Patent: US4416683 A1, 1983 ; |
| Precursor 1 | |
|---|---|
| DownStream 0 | |
|
Coupling passive sampling and time of flight mass spectrometry for a better estimation of polar pesticide freshwater contamination: Simultaneous target quantification and screening analysis.
J. Chromatogr. A. 1387 , 75-85, (2015) The aim of this study was first to develop and validate an analytical method for the quantification of 35 polar pesticides and 9 metabolites by ultra-high-performance-liquid chromatography combined wi... |
|
|
A fluorescent hormone biosensor reveals the dynamics of jasmonate signalling in plants.
Nat. Commun. 6 , 6043, (2015) Activated forms of jasmonic acid (JA) are central signals coordinating plant responses to stresses, yet tools to analyse their spatial and temporal distribution are lacking. Here we describe a JA perc... |
|
|
Toxicity of herbicides in highway runoff.
Environ. Toxicol. Chem. 24(9) , 2336-40, (2005) Previous field monitoring at two highway sites found highway-applied herbicides in storm water runoff at maximum concentrations ranging from 10 microg/L for glyphosate and diuron to as high as 200 mic... |
| 2,6-dimethoxy-N-[3-(3-methylpentan-3-yl)-1,2-oxazol-5-yl]benzamide |
| Caswell No. 419F |
| N-[3-(1-ethyl-1-methylpropyl)-5-isoxazolyl]-2,6-dimethoxybenzamide |
| benzamizole |
| Cent 7 |
| Isoxaben |
| EINECS 407-190-8 |
| Flexidor |
| MFCD00078687 |
| X-Pand |
| Gallery |
| N-[3-(1-ethyl-1-methylpropyl)-1,2-oxazol-5-yl]-2,6-dimethoxybenzamide |
| ixoxaben |