Deoxycholic acid
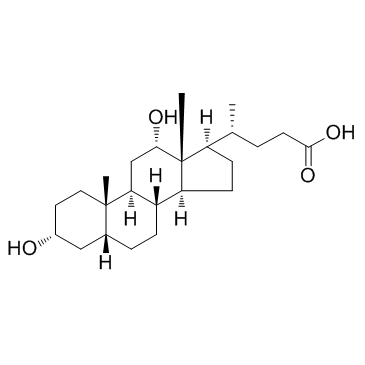
Deoxycholic acid structure
|
Common Name | Deoxycholic acid | ||
|---|---|---|---|---|
| CAS Number | 83-44-3 | Molecular Weight | 392.572 | |
| Density | 1.1±0.1 g/cm3 | Boiling Point | 547.1±35.0 °C at 760 mmHg | |
| Molecular Formula | C24H40O4 | Melting Point | 171-174 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 298.8±22.4 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of Deoxycholic acidDeoxycholic acid is specifically responsible for activating the G protein-coupled bile acid receptor TGR5 that stimulates brown adipose tissue (BAT) thermogenic activity. |
| Name | Deoxycholic Acid |
|---|---|
| Synonym | More Synonyms |
| Description | Deoxycholic acid is specifically responsible for activating the G protein-coupled bile acid receptor TGR5 that stimulates brown adipose tissue (BAT) thermogenic activity. |
|---|---|
| Related Catalog | |
| Target |
Human Endogenous Metabolite |
| In Vitro | Deoxycholic acid (DCA) and chenoDeoxycholic acid (CDCA), as the common ingredients of duodenal reflux, act synergistically in many physiological and pathological processes. The cells are repeatedly exposed to 100 μM CDCA and Deoxycholic acid at pH 5.5 for up to 120 min. To simulate chronic local recurrent disease in vitro, the gastric cancer cell line MGC803 is exposed to acidified medium (pH 5.5) containing 100 μM Deoxycholic acid and CDCA. An untreated log-growth MGC803 cell line is generated to be used as a control in normal pH media. After daily 10 min exposure to the acidified bile acids for 60 weeks, MGC803-resistant cells are able to survive and proliferate after 120 min exposure[2]. |
| Cell Assay | MGC803 cells are cultured in Roswell Park Memorial Institute media supplemented with 10% fetal calf serum and 100 U/mL Penicillin and 100 mg/mL Streptomycin. To generate MGC803-resistant cells, the pH value of the MGC803 culture medium is adjusted to the experimental conditions using the hydrochloric acid (A). The bile acids GCDA and Deoxycholic acid are diluted to optimal working concentrations of 100 μM (B) with culture medium, and the overall pH (A+B) is adjusted to pH 5.5, simulating the gastric environment. Initially, MGC803 cells are chronically exposed to acidified medium with bile acids (A+B) for 10 min every 24 h. The experimental time and conditions are optimized in our preliminary experiments, which show that 10 min is enough and does not result in cell damage. This procedure is repeated and it takes 60 weeks for the MGC803 cells to survive and proliferate under the exposure of A+B for 120 min. Control untreated cells are cultured in neutral RPMI medium at pH 7.4 in parallel to the resistant cells for 60 weeks. The morphological changes in MGC803 cells exposed to acidified bile acids (A+B) are documented at 30 and 60 weeks[2]. |
| References |
| Density | 1.1±0.1 g/cm3 |
|---|---|
| Boiling Point | 547.1±35.0 °C at 760 mmHg |
| Melting Point | 171-174 °C(lit.) |
| Molecular Formula | C24H40O4 |
| Molecular Weight | 392.572 |
| Flash Point | 298.8±22.4 °C |
| Exact Mass | 392.292664 |
| PSA | 77.76000 |
| LogP | 4.66 |
| Vapour Pressure | 0.0±3.3 mmHg at 25°C |
| Index of Refraction | 1.543 |
| Water Solubility | 0.24 g/L (15 ºC) |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302 |
| Precautionary Statements | P301 + P312 + P330 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xn:Harmful |
| Risk Phrases | R22;R36/37/38 |
| Safety Phrases | S26-S36-S37/39 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | FZ2100000 |
| Precursor 9 | |
|---|---|
| DownStream 8 | |
|
MicroRNA-99a and 100 mediated upregulation of FOXA1 in bladder cancer.
Oncotarget 5(15) , 6375-86, (2014) Urothelial cell carcinoma of the bladder (UCC) is a common disease often characterized by FGFR3 dysregulation. Whilst upregulation of this oncogene occurs most frequently in low-grade non-invasive tum... |
|
|
Involvement of epigenetics and EMT-related miRNA in arsenic-induced neoplastic transformation and their potential clinical use.
Cancer Prev. Res. (Phila.) 8(3) , 208-21, (2015) Exposure to toxicants leads to cumulative molecular changes that overtime increase a subject's risk of developing urothelial carcinoma. To assess the impact of arsenic exposure at a time progressive m... |
|
|
MicroRNA expression profiles associated with acquired gefitinib-resistance in human lung adenocarcinoma cells.
Mol. Med. Report. 11(1) , 333-40, (2014) The aim of the present study was to establish, characterize and elucidate the potential mechanisms of acquired gefitinb resistance, using the A549 human lung cancer cell line. A gefitinib-resistant A5... |
| 7-DEOXYCHOLIC ACID |
| Cholan-24-oic acid, 3,12-dihydroxy-, (3α,5β,12α)- |
| 5b-Deoxycholic acid |
| Deoxycholatic acid |
| EINECS 201-478-5 |
| Deoxycholic acid |
| 17b-(1-Methyl-3-carboxypropyl)etiocholane-3a,12a-diol |
| deoxy-Cholic acid |
| (3α,5β,12α)-3,12-Dihydroxycholan-24-oic acid |
| 7α-Deoxycholic acid |
| Kybella |
| 3α,12α-dihydroxy-5β-cholan-24-oic acid |
| ATX-101 |
| 5b-Cholanic acid-3a,12a-diol |
| Dihydroxycholanoic acid |
| Cholic acid, deoxy- |
| (3a,5b,12a)-3,12-Dihydroxy-5-cholan-24-oic Acid |
| (4R)-4-[(3R,5R,8R,9S,10S,12S,13R,14S,17R)-3,12-Dihydroxy-10,13-dimethylhexadecahydro-1H-cyclopenta[a]phenanthren-17-yl]pentanoic acid |
| MFCD00003673 |
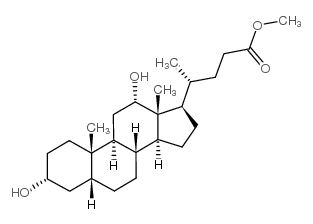 CAS#:3245-38-3
CAS#:3245-38-3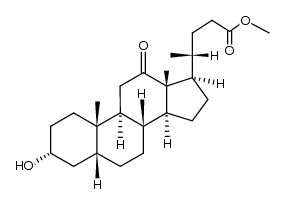 CAS#:28050-47-7
CAS#:28050-47-7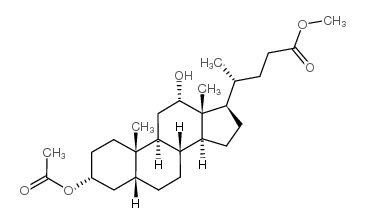 CAS#:27240-83-1
CAS#:27240-83-1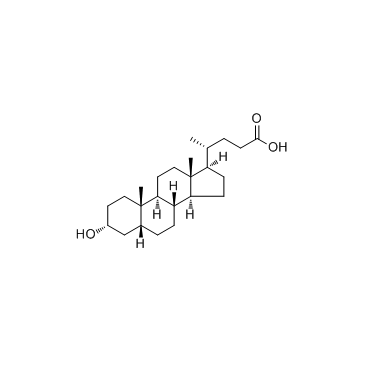 CAS#:434-13-9
CAS#:434-13-9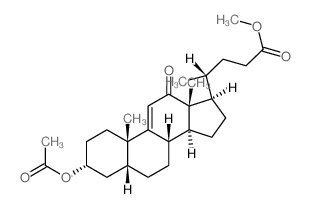 CAS#:4472-02-0
CAS#:4472-02-0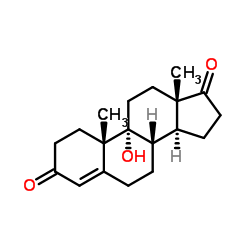 CAS#:560-62-3
CAS#:560-62-3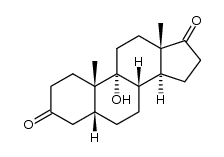 CAS#:1093397-61-5
CAS#:1093397-61-5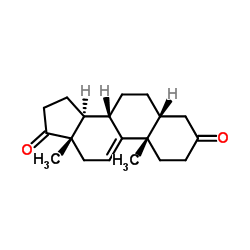 CAS#:1093397-63-7
CAS#:1093397-63-7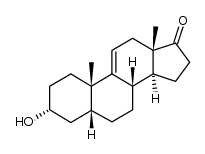 CAS#:571-49-3
CAS#:571-49-3 CAS#:360-65-6
CAS#:360-65-6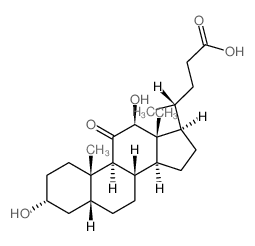 CAS#:15173-30-5
CAS#:15173-30-5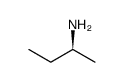 CAS#:513-49-5
CAS#:513-49-5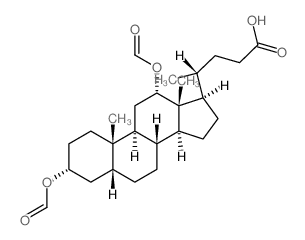 CAS#:2287-93-6
CAS#:2287-93-6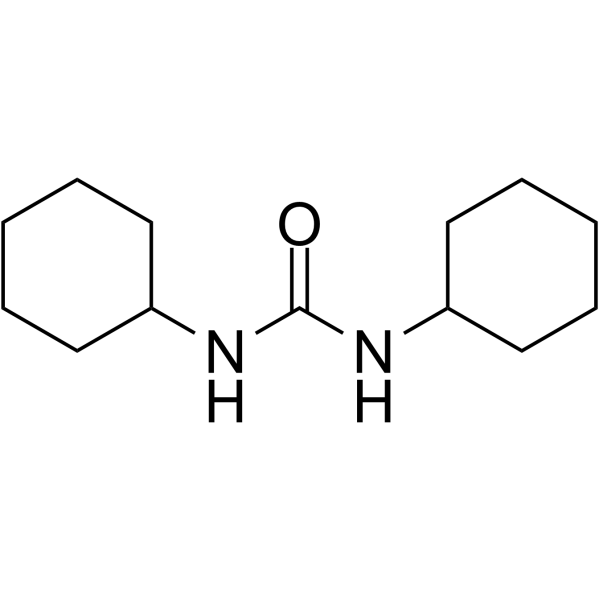 CAS#:2387-23-7
CAS#:2387-23-7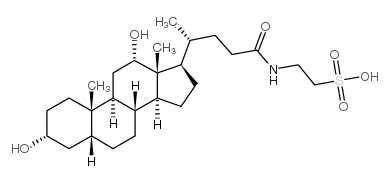 CAS#:516-50-7
CAS#:516-50-7
