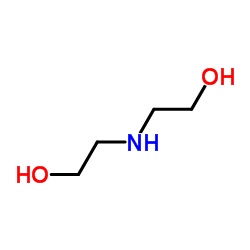
Treprostinil (diethanolamine salt)
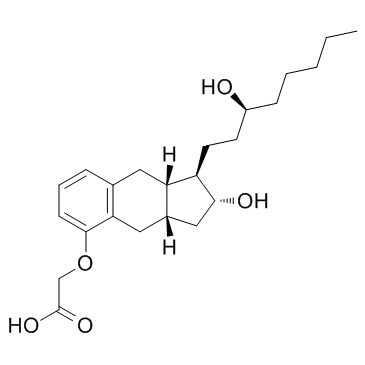
Treprostinil (diethanolamine salt) structure
|
Common Name | Treprostinil (diethanolamine salt) | ||
|---|---|---|---|---|
| CAS Number | 830354-48-8 | Molecular Weight | 495.64900 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C27H45NO7 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | N/A | |
Use of Treprostinil (diethanolamine salt)Treprostinil (UT-15C) diethanolamine is a potent EP2, DP1 and IP agonist with Ki values of 3.6, 4.4, 32.1, 212, 826, 2505 and 4680 nM for EP2, DP1, IP, EP1, EP4, EP3 and FP, respectively. Treprostinil (UT-15C) diethanolamine increases upregulation of cAMP toward maintaining homeostasis within the vasculature. Treprostinil (UT-15C) diethanolamine can result in vasodilatation of human pulmonary arteries[1][2][3]. |
| Name | 2-[[(1R,2R,3aS,9aS)-2-hydroxy-1-[(3S)-3-hydroxyoctyl]-2,3,3a,4,9,9a-hexahydro-1H-cyclopenta[g]naphthalen-5-yl]oxy]acetic acid,2-(2-hydroxyethylamino)ethanol |
|---|---|
| Synonym | More Synonyms |
| Description | Treprostinil (UT-15C) diethanolamine is a potent EP2, DP1 and IP agonist with Ki values of 3.6, 4.4, 32.1, 212, 826, 2505 and 4680 nM for EP2, DP1, IP, EP1, EP4, EP3 and FP, respectively. Treprostinil (UT-15C) diethanolamine increases upregulation of cAMP toward maintaining homeostasis within the vasculature. Treprostinil (UT-15C) diethanolamine can result in vasodilatation of human pulmonary arteries[1][2][3]. |
|---|---|
| Related Catalog | |
| Target |
DP/DP1:4.4 nM (Ki) IP:32.1 nM (Ki) EP1:212 nM (Ki) EP2:3.6 nM (Ki) EP3:826 nM (Ki) EP4:2505 nM (Ki) FP:4680 nM (Ki) |
| In Vitro | Treprostinil (UT-15C) diethanolamine (0.001-10,000 nM; 60 min; HEK293 cells) has high potency in activating DP1 and EP2 receptors as well as the IP receptor with EC50 values of 0.6 nM, 6.2 nM and 1.9 nM, respectively, 36-fold less active at the EP3 receptor, 95-fold less active at the EP4 and 150-fold less active at the EP1 site than at the IP receptor[1]. Treprostinil (UT-15C) diethanolamine (10 μM) increases cAMP accumulation in murine and human hematopoietic stem and progenitor cells (HSPCs) [2]. Treprostinil (UT-15C) diethanolamine (10 μM; 2-6 h; PC3 cells) enhances the action of SDF-1 via CXCR4[2]. |
| In Vivo | Treprostinil (UT-15C) diethanolamine (0.15 mg/kg; i.h.; every 8 h; for 10 d; BALB/c mice) enhances the ability of HSPCs to repopulate the bone marrow and increases bone marrow reconstitution[2]. Treprostinil (UT-15C) diethanolamine (0.15 mg/kg; i.h.; every 8 h; for 10 d; BALB/c mice) increases survival of lethally irradiated recipient mice[2]. Treprostinil (UT-15C) diethanolamine (0.1 mg/kg; i.h.; for 24h; male lewis rats) inhibits the mRNA expression of TNF-α and IFN-γ and increases in IL-10 expression[3]. Animal Model: BALB/c mice[2] Dosage: 0.15 mg/kg Administration: Subcutaneous injection; every 8 hours; for 10 days Result: Increased survival of lethally irradiated recipient mice. Animal Model: Male lewis rats[3] Dosage: 0.1 mg/kg Administration: Subcutaneous injection; for 24 hours Result: Decreased the mRNA expression of TNF-α and IFN-γ and increased the expression of IL-10. |
| References |
| Molecular Formula | C27H45NO7 |
|---|---|
| Molecular Weight | 495.64900 |
| Exact Mass | 495.32000 |
| PSA | 146.89000 |
|
~80% 
Treprostinil (d... CAS#:830354-48-8 |
| Literature: UNITED THERAPEUTICS CORPORATION Patent: US2009/163738 A1, 2009 ; Location in patent: Page/Page column 6-7 ; |
| Precursor 2 | |
|---|---|
| DownStream 1 | |
| Treprostinil diethanolamine |
| TREPROSTINIL DIOLAMINE |
| UT-15C |
| treprostinil diethanolamine salt |