Vapreotide acetate
Modify Date: 2024-01-02 20:53:42
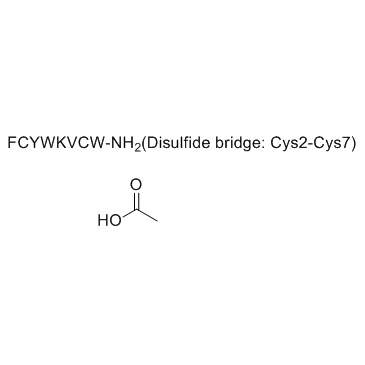
Vapreotide acetate structure
|
Common Name | Vapreotide acetate | ||
|---|---|---|---|---|
| CAS Number | 849479-74-9 | Molecular Weight | 1209.438 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C57H70N12O9S2.xC2H4O2 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | N/A | |
Use of Vapreotide acetateVapreotide acetate is a synthetic analog of somatostatin for the treatment of variceal bleeding; also exhibits antitumor activity. |
| Name | Vapreotide acetate |
|---|---|
| Synonym | More Synonyms |
| Description | Vapreotide acetate is a synthetic analog of somatostatin for the treatment of variceal bleeding; also exhibits antitumor activity. |
|---|---|
| Related Catalog | |
| In Vitro | Somatostatin is a peptide hormone which is distributed in many tissues including the immune system and the nervous system. Vapreotide attenuates the Substance P (SP)-triggered intracellular calcium increases and NF-κB activation in a dose-dependent manner. Vapreotide also inhibits SP-induced IL-8 and MCP-1 production in HEK293-NK1R and U373MG cell lines. Vapreotide inhibits HIV-1 infection of human MDM in vitro, an effect that is reversible by SP pretreatment[1]. Vapreotide significantly inhibits GH-, PRL, and/or alpha-subunit release by human GH-secreting pituitary adenoma cells in concentrations as low as 10-12-10-14 M. Vapreotide inhibits GH release with an IC50 of 0.1 pM[2]. Vapreotide exhibits moderate-to-high affinities for SSTR2, -3, and -5 (IC50=0.17, 0.1 and 21 nM, respectively) and low affinity for SSTR1 and -4 (IC50=200 and 620 nM, respectively). RC-160 inhibits serum-induced proliferation of CHO cells expressing SSTR2 and SSTR5 (EC50=53 and 150 pM, respectively)[3]. |
| In Vivo | In cirrhosis, bleeding by rupture of oesophagogastric varices is a severe complication of portal hypertension. The acute administration of vapreotide to rats decreases collateral circulation blood flow while chronic administration attenuated its development[4]. Tumor volumes and weights are reduced by about 40% by RC-160 by s.c. injections at doses of 100 μg/day/animal. Vapreotide can inhibit the growth of androgen-independent prostate cancer when the therapy is started at an early stage of tumor development[5]. |
| Cell Assay | CHO cells are cultured in aMEM containing 10% FCS. After overnight attachment, the medium is changed to a MEM containing either 10% FCS or insulin with and without vapreotide. Cell growth is measured after 24 h by counting cells with a Coulter counter model ZM[3]. |
| Animal Admin | Rats: Acute effects are evaluated at baseline and 30 min after placebo or vapreotide (8 μg/kg/hr) infusions in DMNA rats. Chronic hemodynamic effects are evaluated using subcutaneous implants for five weeks in anesthetized DMNA rats and in sham rats. Hemodynamic measurements include splenorenal shunt blood flow by the transit time ultrasound method and cardiac output by the combined dilution–TTU method[4]. Mice: Nude mice bearing xenografts of the androgen-independent human prostate-cancer cell line PC-3 are treated for 4 weeks with somatostatin analog vapreotide (20 μg/day/animal), bombesin/gastrin-releasing peptide (GRP) antagonist, or the combination of both peptides. Tumor volumes and weights are measured[5]. |
| References |
| Molecular Formula | C57H70N12O9S2.xC2H4O2 |
|---|---|
| Molecular Weight | 1209.438 |
| Exact Mass | 1208.514648 |
| 1H-Indole-3-propanamide, α-[[[(4R,7S,10S,13R,16S,19R)-10-(4-aminobutyl)-19-[[(2R)-2-amino-1-oxo-3-phenylpropyl]amino]-16-[(4-hydroxyphenyl)methyl]-13-(1H-indol-3-ylmethyl)-7-(1-methylethyl)-6,9,12 ,15,18-pentaoxo-1,2-dithia-5,8,11,14,17-pentaazacycloeicos-4-yl]carbonyl]amino]-, (αS)-, acetate, hydrate (1:1:1) (salt) |
| (4R,7S,10S,13R,16S,19R)-10-(4-Aminobutyl)-N-[(2S)-1-amino-3-(1H-indol-3-yl)-1-oxo-2-propanyl]-16-(4-hydroxybenzyl)-13-(1H-indol-3-ylmethyl)-7-isopropyl-6,9,12,15,18-pentaoxo-19-(D-phenylalanylamino)-1,2-dithia-5,8,11,14,17-pentaazacycloicosane-4-carboxamide acetate hydrate (1:1:1) |