4,5,6,7-Tetrachloro-2-hydroxy-isoindole-1,3-dione
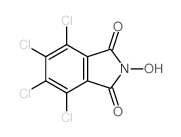
4,5,6,7-Tetrachloro-2-hydroxy-isoindole-1,3-dione structure
|
Common Name | 4,5,6,7-Tetrachloro-2-hydroxy-isoindole-1,3-dione | ||
|---|---|---|---|---|
| CAS Number | 85342-65-0 | Molecular Weight | 300.91000 | |
| Density | 2.041g/cm3 | Boiling Point | 532.1ºC at 760mmHg | |
| Molecular Formula | C8HCl4NO3 | Melting Point | N/A | |
| MSDS | Chinese | Flash Point | 275.6ºC | |
| Symbol |

GHS06 |
Signal Word | Danger | |
| Name | 4,5,6,7-tetrachloro-2-hydroxyisoindole-1,3-dione |
|---|---|
| Synonym | More Synonyms |
| Density | 2.041g/cm3 |
|---|---|
| Boiling Point | 532.1ºC at 760mmHg |
| Molecular Formula | C8HCl4NO3 |
| Molecular Weight | 300.91000 |
| Flash Point | 275.6ºC |
| Exact Mass | 298.87100 |
| PSA | 57.61000 |
| LogP | 3.22330 |
| Index of Refraction | 1.729 |
| Symbol |

GHS06 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H301 |
| Precautionary Statements | Missing Phrase - N15.00950417 |
| RIDADR | UN 2811 6.1 / PGIII |
|
~98% 
4,5,6,7-Tetrach... CAS#:85342-65-0 |
| Literature: Sugamoto, Kazuhiro; Matsushita, Yoh-Ichi; Kameda, Yu-Hei; Suzuki, Masahiko; Matsui, Takanao Synthetic Communications, 2005 , vol. 35, # 1 p. 67 - 70 |
|
~% 
4,5,6,7-Tetrach... CAS#:85342-65-0 |
| Literature: Einhorn; Marcadal-Abbadi Synthetic Communications, 2001 , vol. 31, # 5 p. 741 - 748 |
| Precursor 2 | |
|---|---|
| DownStream 0 | |
|
Efficient metal-free aerobic oxidation of aromatic hydrocarbons utilizing aryl-tetrahalogenated N-hydroxyphthalimides and 1,4-diamino-2,3-dichloroanthraquinone. Zhang Q, et al.
J. Chem. Technol. Biotechnol. 83(10) , 1364-1369, (2008)
|
|
|
Nickel-Catalyzed Cross-Coupling of Redox-Active Esters with Boronic Acids.
Angew. Chem. Int. Ed. Engl. 55(33) , 9676-9, (2016) A transformation analogous in simplicity and functional group tolerance to the venerable Suzuki cross-coupling between alkyl-carboxylic acids and boronic acids is described. This Ni-catalyzed reaction... |
|
|
A general alkyl-alkyl cross-coupling enabled by redox-active esters and alkylzinc reagents.
Science 352(6287) , 801-5, (2016) Alkyl carboxylic acids are ubiquitous in all facets of chemical science, from natural products to polymers, and represent an ideal starting material with which to forge new connections. This study dem... |
| N-hydroxy-3,4,5,6-tetrachlorophthalimide |
| 4,5,6,7-Tetrachlor-2-hydroxy-isoindolin-1,3-dion |
| N-hydroxytetrachlorophtalimide |
| 3,4,5,6-tetrachloro-N-hydroxyphthalimide |
| 4,5,6,7-tetrachloro-2-hydroxy-isoindole-1,3-dione |
| 4,5,6,7-tetrachloro-2-hydroxy-isoindoline-1,3-dione |
| N-hydroxytetrachlorophthalimide |
| tetrachloro-N-hydroxyphthalimide |