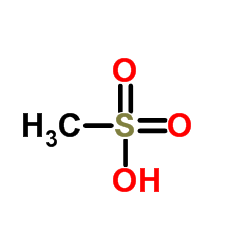
Lenvatinib Mesylate
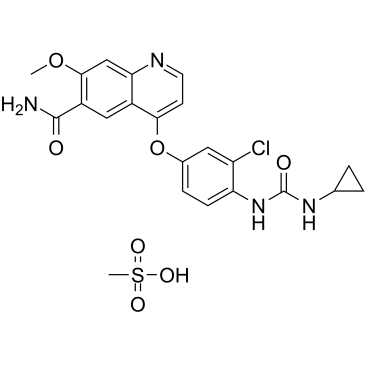
Lenvatinib Mesylate structure
|
Common Name | Lenvatinib Mesylate | ||
|---|---|---|---|---|
| CAS Number | 857890-39-2 | Molecular Weight | 522.959 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C22H23ClN4O7S | Melting Point | N/A | |
| MSDS | N/A | Flash Point | N/A | |
Use of Lenvatinib MesylateLenvatinib mesylate (E7080 mesylate), an oral, multi-targeted tyrosine kinase inhibitor that inhibits VEGFR1-3, FGFR1-4, PDGFR, KIT, and RET, shows potent antitumor activities[1][2]. |
| Name | lenvatinib mesylate |
|---|---|
| Synonym | More Synonyms |
| Description | Lenvatinib mesylate (E7080 mesylate), an oral, multi-targeted tyrosine kinase inhibitor that inhibits VEGFR1-3, FGFR1-4, PDGFR, KIT, and RET, shows potent antitumor activities[1][2]. |
|---|---|
| Related Catalog | |
| Target |
VEGFR1:22 nM (IC50) VEGFR2:4 nM (IC50) VEGFR3:5.2 nM (IC50) FGFR1:46 nM (IC50) FGFR2 FGFR3 FGFR4 PDGFRα:51 nM (IC50) PDGFRβ:39 nM (IC50) c-Kit:100 nM (IC50) RET |
| In Vitro | Lenvatinib mesylate (E7080 mesylate) has IC50s of 4, 5.2, 22 nM for VEGFR2(KDR), VEGFR3(Flt-4), and VEGFR1/Flt-1, respectively. Lenvatinib inhibits PDGFRα, PDGFRβ, FGFR1, and KIT with IC50s of 51, 39, 46, 100 nM, respectively[3]. |
| In Vivo | Lenvatinib mesylate (E7080 mesylate) (100 mg/kg, p.o.) is administeredand bevacizumab significantly inhibits local tumor growth at the m.f.p., and at the end of treatment, Lenvatinib mesylate also significantly inhibits metastasis to both regional lymph nodes and distant lung[3]. Lenvatinib mesylate (E7080 mesylate) inhibits the growth of H146 tumor at 30 and 100 mg/kg (BID, QDx21) in a dose-dependent manner and causes tumor regression at 100 mg/kg in H146 xenograft model. IHC analysis with anti-CD31 antibody shows that lenvatinib at 100 mg/kg decreases microvessel density more than anti-VEGF antibody and imatinib treatment[4]. |
| References |
| Molecular Formula | C22H23ClN4O7S |
|---|---|
| Molecular Weight | 522.959 |
| Exact Mass | 522.097595 |
| PSA | 182.80000 |
| LogP | 5.81830 |
|
~% 
Lenvatinib Mesylate CAS#:857890-39-2 |
| Literature: Eisai Co., Ltd. Patent: EP1698623 A1, 2006 ; Location in patent: Page/Page column 17 ; |
| 4-(3-chloro-4-(cyclopropylaminocarbonyl)aminophenoxy)-7-methoxy-6-quinolinecarboxamide mesylate |
| Lenvatinib mesilate |
| lenvatinib mesylate |
| Lenvatinib mesylate [USAN] |
| 4-{3-Chloro-4-[(cyclopropylcarbamoyl)amino]phenoxy}-7-methoxy-6-quinolinecarboxamide methanesulfonate (1:1) |
| 4-(3-chloro-4-(cyclopropylaminocarbonyl)aminophenoxy)-7-methoxy-6-quinolinecarboxamide methanesulfonate |
| 4-[3-chloro-4-(cyclopropylcarbamoylamino)phenoxy]-7-methoxyquinoline-6-carboxamide,methanesulfonic acid |
| 6-Quinolinecarboxamide, 4-[3-chloro-4-[[(cyclopropylamino)carbonyl]amino]phenoxy]-7-methoxy-, methanesulfonate (1:1) |
| Lenvatinib mesilate (JAN) |
| lenvatinib methanesulfonate |
| UNII-3J78384F61 |