| Description |
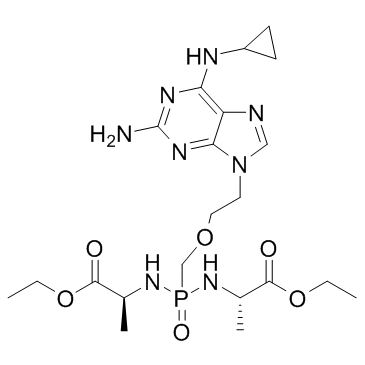
Rabacfosadine (GS-9219), a novel prodrug of the nucleotide analogue PMEG, is designed as a cytotoxic agent that preferentially targets lymphoid cells.
|
| Related Catalog |
|
| In Vitro |
In lymphocytes, Rabacfosadine (GS-9219) is converted to its active metabolite, 9-(2-phosphonylmethoxyethyl)guanine (PMEG) diphosphate, via enzymatic hydrolysis, deamination, and phosphorylation. GS-9219 has substantial antiproliferative activity against activated lymphocytes and hematopoietic tumor cell lines. The ability of Rabacfosadine to inhibit the proliferation of activated lymphocytes and of tumor cells of hematopoietic origin is investigated. Rabacfosadine inhibits the proliferation of mitogen-stimulated T and B lymphocytes with EC50 values of 135 and 42 nM, respectively, as determined by BrdUrd incorporation. To compare the activity of GS-9219 in dividing and nondividing cells, Rabacfosadine is evaluated in these populations using a metabolism-based sodium XTT assay instead of BrdUrd assays. Results from the XTT assay shows a 127-fold difference between the EC50 values of Rabacfosadine in quiescent (EC50=17.2 μM) and proliferating (EC50=135 nM) cells. These results indicate a substantial selectivity of Rabacfosadine toward actively replicating lymphoblasts[1].
|
| In Vivo |
Rabacfosadine (RAB) has substantial single-agent activity in dogs with lymphoma, and a different mechanism of action than Doxorubicin (DOX). Open-label, multicenter prospective clinical trial. Dogs receive alternating Rabacfosadine (1.0 mg/kg IV weeks 0, 6, 12) and Doxorubicin (30 mg/m2 IV weeks 3, 9, 15). Dogs that achieved complete response (CR) are followed by monthly evaluations. Complete clinicopathological evaluation and assessment of remission and adverse event (AEs) are performed every 21 days. Acute AEs, occurring within 21 days after administration of the first dose of each agent, are compared between Rabacfosadine and Doxorubicin in 46 dogs receiving at least 1 dose of each agent[2].
|
| Kinase Assay |
Twenty million PHA-stimulated T cells are incubated with 10 μM[14C]GS-9219 for 24 h. Cells are washed once with tissue culture medium and twice with PBS, incubated in 80% methanol overnight, and centrifuged at 14,000 rpm for 15 min to remove denatured proteins. The methanol extracts are lyophilized and dissolved in the high-performance liquid chromatography (HPLC) loading buffer. A portion of each sample is used for scintillation counting to calculate the total pmoles, and the rest is used for HPLC analysis to calculate the ratio of metabolites. HPLC analysis is done by gradient elution using a Phenomenex Prodigy column (5 μm, ODS3 150×4 mm), where buffer A is 25 mM K2HPO4 (pH 6.0) and 5 mM Tetrabutylammonium bromide and buffer B is 25 mM K2HPO4 (pH 6.0), 70% Acetonitrile, and 5 mM Tetrabutylammonium bromide[1].
|
| Animal Admin |
Dogs[2] Dogs are given alternating doses of Rabacfosadine and Doxorubicin every 3 weeks. Rabacfosadine is administered at a dosage of 1.0 mg/kg as a 30-minute IV infusion on weeks 0, 6, and 12. Doxorubicin is administered at a dosage of 30 mg/m2 (1.0 mg/kg for dogs weighing <15 kg) as an approximately 20-minute IV infusion on weeks 3, 9, and 15. Concurrent cytotoxic chemotherapy of any kind and concurrent corticosteroids are not allowed. A CBC is performed 1 week after the first Rabacfosadine and Doxorubicin treatments (weeks 1 and 4). All dogs have a physical examination with lymph node measurements, owner history, CBC, serum biochemistry profile (SBC), and urinalysis performed at each chemotherapy visit. Thoracic radiographs are recommended at week 15 and approximately every 2 months thereafter in responding dogs. Dose delays, reductions, or both, and supportive treatment for AEs are carried out at the discretion of the attending clinician. Dogs that experienced a complete response (CR) are followed with monthly physical examinations after week 15 until relapse, when they are considered off study. Dogs that experienced partial response (PR) or stable disease (SD) at week 15 are considered off study and censored from analysis at that time. At the time of study withdrawal, dogs are eligible for additional treatment at the discretion of the attending clinician.
|
| References |
[1]. Reiser H, et al. GS-9219--a novel acyclic nucleotide analogue with potent antineoplastic activity in dogs with spontaneous non-Hodgkin's lymphoma. Clin Cancer Res. 2008 May 1;14(9):2824-32. [2]. Thamm DH, et al. Alternating Rabacfosadine/Doxorubicin: Efficacy and Tolerability in Naïve Canine Multicentric Lymphoma. J Vet Intern Med. 2017 May;31(3):872-878.
|