4,7-Dichloroquinoline
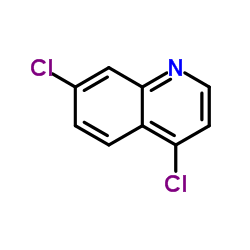
4,7-Dichloroquinoline structure
|
Common Name | 4,7-Dichloroquinoline | ||
|---|---|---|---|---|
| CAS Number | 86-98-6 | Molecular Weight | 198.05 | |
| Density | 1.4±0.1 g/cm3 | Boiling Point | 292.9±20.0 °C at 760 mmHg | |
| Molecular Formula | C9H5Cl2N | Melting Point | 81-83 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 158.7±7.4 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of 4,7-Dichloroquinoline4,7-Dichloroquinoline is a biochemical reagent that can be used as a biological material or organic compound for life science related research. |
| Name | 4,7-Dichloroquinoline |
|---|---|
| Synonym | More Synonyms |
| Description | 4,7-Dichloroquinoline is a biochemical reagent that can be used as a biological material or organic compound for life science related research. |
|---|---|
| Related Catalog |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 292.9±20.0 °C at 760 mmHg |
| Melting Point | 81-83 °C(lit.) |
| Molecular Formula | C9H5Cl2N |
| Molecular Weight | 198.05 |
| Flash Point | 158.7±7.4 °C |
| Exact Mass | 196.979904 |
| PSA | 12.89000 |
| LogP | 3.46 |
| Vapour Pressure | 0.0±0.6 mmHg at 25°C |
| Index of Refraction | 1.661 |
| Water Solubility | insoluble |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H319-H335 |
| Precautionary Statements | P261-P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xi:Irritant |
| Risk Phrases | R36/37/38 |
| Safety Phrases | S26-S36-S22 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | VB4200000 |
| HS Code | 2933499013 |
| Precursor 9 | |
|---|---|
| DownStream 10 | |
| HS Code | 2933499090 |
|---|---|
| Summary | 2933499090. other compounds containing in the structure a quinoline or isoquinoline ring-system (whether or not hydrogenated), not further fused. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
New bioactive compounds from Aloe hijazensis.
Nat. Prod. Res. 23 , 1035-1049, (2009) The chemical constituents and biological activities of leaves and roots of Aloe hijazensis, collected in Saudi Arabia, are reported here for the first time. Twenty-two compounds were obtained, among t... |
|
|
Direct, catalytic, and regioselective synthesis of 2-alkyl-, aryl-, and alkenyl-substituted N-heterocycles from N-oxides.
Org. Lett. 16(3) , 864-7, (2014) A one-step transformation of heterocyclic N-oxides to 2-alkyl-, aryl-, and alkenyl-substituted N-heterocycles is described. The success of this broad-scope methodology hinges on the combination of cop... |
|
|
"One-pot" synthesis and antimalarial activity of formamidine derivatives of 4-anilinoquinoline.
Chem. Pharm. Bull. 49(8) , 933-7, (2001) Amodiaquine (AQ) is an antimalarial which is effective against chloroquino-resistant strains of Plasmodium falciparum but whose clinical use is severely restricted because of associated hepatotoxicity... |
| Quinoline, 4,7-dichloro- |
| 4,7- Dichloroquinoline |
| 4,7-DICHLORO QUINOLINE |
| 4,7-DICHLORCHINOLIN |
| 4,7-Dichloroquinoline,tech. |
| 4,7-dichloroquinolne |
| 4,7-DICHLOROQUINOLINE FOR SYNTHESIS |
| 7-Dichloroquinoline |
| TL 1473 |
| 4 7-DICHLOROQUINOLINE |
| Chloroquine Related Compound A (25 mg) (4,7-dichloroquinoline) |
| 4,7-Dichloroquinoline |
| 4,7-DICHLOROQUINOLI |
| 4,7-dichloro-quinolin |
| 4.7-dichloroquinoline |
| 4,7-Dichlor-chinolin |
| 4,7-DICHLOLROQUINOLINE |
| EINECS 201-714-7 |
| MFCD00006774 |
 CAS#:86-99-7
CAS#:86-99-7 CAS#:1134937-73-7
CAS#:1134937-73-7 CAS#:108-42-9
CAS#:108-42-9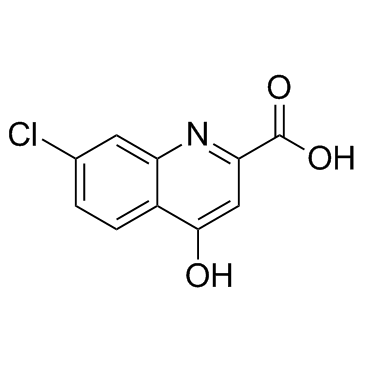 CAS#:18000-24-3
CAS#:18000-24-3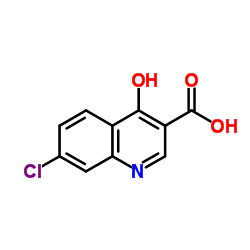 CAS#:86-47-5
CAS#:86-47-5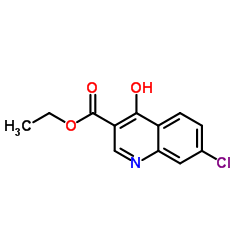 CAS#:16600-22-9
CAS#:16600-22-9![Propanedioic acid,2-[[(3-chlorophenyl)amino]methylene]-, 1,3-diethyl ester Structure](https://image.chemsrc.com/caspic/023/3412-99-5.png) CAS#:3412-99-5
CAS#:3412-99-5 CAS#:25063-49-4
CAS#:25063-49-4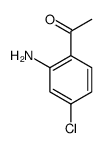 CAS#:39061-72-8
CAS#:39061-72-8![[4-[(7-chloroquinolin-4-yl)amino]phenyl]-morpholin-4-ylmethanone structure](https://image.chemsrc.com/caspic/360/108199-09-3.png) CAS#:108199-09-3
CAS#:108199-09-3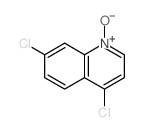 CAS#:1077-74-3
CAS#:1077-74-3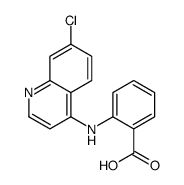 CAS#:10440-42-3
CAS#:10440-42-3![7-Chloro-N-{(3-chlorophenyl)[4-(1-pyrrolidinylmethyl)phenyl]methy l}-4-quinolinamine structure](https://image.chemsrc.com/caspic/142/1050526-88-9.png) CAS#:1050526-88-9
CAS#:1050526-88-9 CAS#:107415-26-9
CAS#:107415-26-9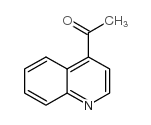 CAS#:60814-30-4
CAS#:60814-30-4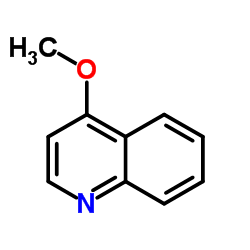 CAS#:607-31-8
CAS#:607-31-8 CAS#:3820-67-5
CAS#:3820-67-5 CAS#:4085-31-8
CAS#:4085-31-8 CAS#:140926-77-8
CAS#:140926-77-8
