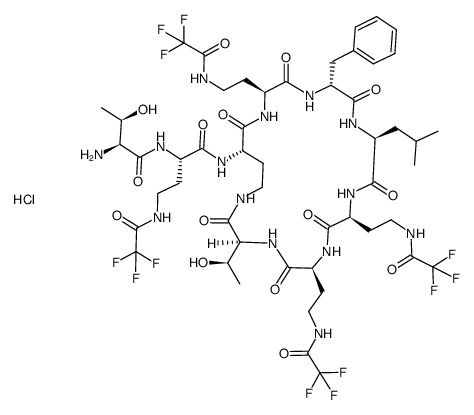
Polymyxin B nonapeptide
Modify Date: 2024-01-06 07:43:29
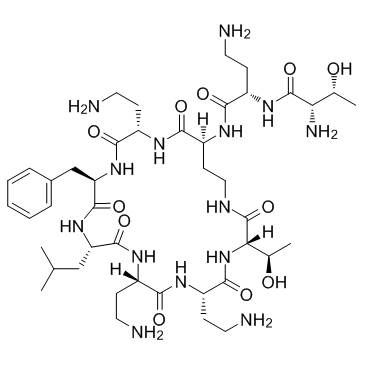
Polymyxin B nonapeptide structure
|
Common Name | Polymyxin B nonapeptide | ||
|---|---|---|---|---|
| CAS Number | 86408-36-8 | Molecular Weight | 963.13500 | |
| Density | 1.32g/cm3 | Boiling Point | 1456.2ºC at 760 mmHg | |
| Molecular Formula | C43H74N14O11 | Melting Point | N/A | |
| MSDS | USA | Flash Point | 834.5ºC | |
Use of Polymyxin B nonapeptidePolymyxin B nonapeptide is a cyclic peptide obtained from Polymyxin B by proteolytic removal of its terminal amino acyl residue[1]. Polymyxin B nonapeptide is less toxic, lacks bactericidal activity, and retains its ability to render gram-negative bacteria susceptible to several antibiotics by permeabilizing their outer membranes[2]. |
| Name | (2S,3R)-2-amino-N-[(2S)-4-amino-1-oxo-1-[[(3S,6S,9S,12S,15R,18S,21S)-6,9,18-tris(2-aminoethyl)-15-benzyl-3-(1-hydroxyethyl)-12-(2-methylpropyl)-2,5,8,11,14,17,20-heptaoxo-1,4,7,10,13,16,19-heptazacyclotricos-21-yl]amino]butan-2-yl]-3-hydroxybutanamide |
|---|---|
| Synonym | More Synonyms |
| Description | Polymyxin B nonapeptide is a cyclic peptide obtained from Polymyxin B by proteolytic removal of its terminal amino acyl residue[1]. Polymyxin B nonapeptide is less toxic, lacks bactericidal activity, and retains its ability to render gram-negative bacteria susceptible to several antibiotics by permeabilizing their outer membranes[2]. |
|---|---|
| Related Catalog | |
| In Vitro | Polymyxin B nonapeptide, a cationic cyclic peptide derived by enzymatic processing from the naturally occurring peptide polymyxin B, is able to increase the permeability of the outer membrane of Gram-negative bacteria toward hydrophobic antibiotics probably by binding to the bacterial lipopolysaccharide (LPS)[1]. |
| References |
| Density | 1.32g/cm3 |
|---|---|
| Boiling Point | 1456.2ºC at 760 mmHg |
| Molecular Formula | C43H74N14O11 |
| Molecular Weight | 963.13500 |
| Flash Point | 834.5ºC |
| Exact Mass | 962.56600 |
| PSA | 463.87000 |
| LogP | 0.27100 |
| Index of Refraction | 1.607 |
| Storage condition | 2-8℃ |
|
~% 
Polymyxin B non... CAS#:86408-36-8 |
| Literature: Okimura, Keiko; Ohki, Kazuhiro; Sato, Yuki; Ohnishi, Kuniharu; Uchida, Yoshiki; Sakura, Naoki Bulletin of the Chemical Society of Japan, 2007 , vol. 80, # 3 p. 543 - 552 |
|
~% 
Polymyxin B non... CAS#:86408-36-8 |
| Literature: Tsubery, Haim; Yaakov, Hertzig; Cohen, Sofia; Giterman, Tal; Matityahou, Ariella; Fridkin, Mati; Ofek, Itzhak Antimicrobial Agents and Chemotherapy, 2005 , vol. 49, # 8 p. 3122 - 3128 |
| triisopropylsilanthiol |
| H-Thr-2,4-diaminobutyryl-cyclo[2,4-diaminobutyryl-2,4-diaminobutyryl-D-Phe-Leu-2,4-diaminobutyryl-2,4-diaminobutyryl-Thr] |
| polymyxin B nonapeptide |
| HSSi(i-Pr)3 |
| TRI-ISOPROPYLSILANETHIOL |
| H-Thr-Dab-cyclic-(Dab-Dab-D-Phe-Leu-Dab-Dab-Thr) |
| polymixin B nonapeptide |
| T-X-cyclo[X-X-DF-L-X-X-T] |