Narlaprevir
Modify Date: 2024-01-02 19:51:11
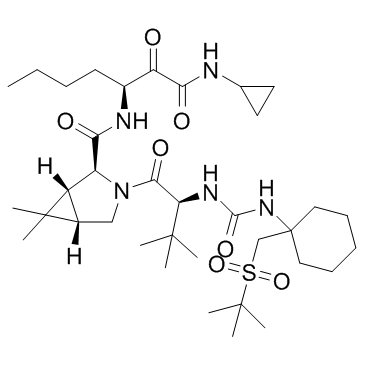
Narlaprevir structure
|
Common Name | Narlaprevir | ||
|---|---|---|---|---|
| CAS Number | 865466-24-6 | Molecular Weight | 707.964 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | N/A | |
| Molecular Formula | C36H61N5O7S | Melting Point | N/A | |
| MSDS | N/A | Flash Point | N/A | |
Use of NarlaprevirNarlaprevir is a potent, selective, orally bioavailable NS3 protease inhibitor(Ki=6 nM; EC90=40 nM)IC50 Value: 6 nM (Ki)Target: HCV NS3/4A Protease; HCVNarlaprevir (SCH 900518) is a potent inhibitor of the hepatitis C virus (HCV) nonstructural protein 3 serine protease that is primarily metabolized by the cytochrome P450-3A4 system. Narlaprevir administration resulted in a robust HCV-RNA decline and high SVR rates when followed by standard of care in both treatment-experienced and treatment-naive HCV genotype 1-infected patients. |
| Name | (1R,2S,5S)-3-[(2S)-2-[[1-(tert-butylsulfonylmethyl)cyclohexyl]carbamoylamino]-3,3-dimethylbutanoyl]-N-[(3S)-1-(cyclopropylamino)-1,2-dioxoheptan-3-yl]-6,6-dimethyl-3-azabicyclo[3.1.0]hexane-2-carboxamide |
|---|---|
| Synonym | More Synonyms |
| Description | Narlaprevir is a potent, selective, orally bioavailable NS3 protease inhibitor(Ki=6 nM; EC90=40 nM)IC50 Value: 6 nM (Ki)Target: HCV NS3/4A Protease; HCVNarlaprevir (SCH 900518) is a potent inhibitor of the hepatitis C virus (HCV) nonstructural protein 3 serine protease that is primarily metabolized by the cytochrome P450-3A4 system. Narlaprevir administration resulted in a robust HCV-RNA decline and high SVR rates when followed by standard of care in both treatment-experienced and treatment-naive HCV genotype 1-infected patients. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Molecular Formula | C36H61N5O7S |
| Molecular Weight | 707.964 |
| Exact Mass | 707.429199 |
| PSA | 186.21000 |
| LogP | 3.61 |
| Index of Refraction | 1.558 |
| (1R,2S,5S)-3-((S)-2-(3-(1-(tert-butylsulfonylmethyl)cyclohexyl)ureido)-3,3-dimethylbutanoyl)-N-((S)-1-(cyclopropylamino)-1,2-dioxoheptan-3-yl)-6,6-dimethyl-3-azabicyclo[3.1.0]hexane-2-carboxamide |
| (1R,2S,5S)-N-[(3S)-1-(Cyclopropylamino)-1,2-dioxo-3-heptanyl]-6,6-dimethyl-3-{3-methyl-N-[(1-{[(2-methyl-2-propanyl)sulfonyl]methyl}cyclohexyl)carbamoyl]-L-valyl}-3-azabicyclo[3.1.0]hexane-2-carboxamide |
| CS-0445 |
| Narlaprevir (USAN/INN) |
| Narlaprevir |
| (1R,5S)-N-[1(S)-[2-(cyclopropylamino)-1,2-dioxoethyl]pentyl]-3-[2(S)-[[[[1-[[(1,1-dimethylethyl)sulfonyl]methyl]cyclohexyl]amino]carbonyl]amino]-3,3-dimethyl-1-oxobutyl]-6,6-dimethyl-3-azabicyclo[3.1.0]hexane-2(S)-carboxamide |
| 3-Azabicyclo[3.1.0]hexane-2-carboxamide, N-[(1S)-1-[2-(cyclopropylamino)-1,2-dioxoethyl]pentyl]-3-[(2S)-2-[[[[1-[[(1,1-dimethylethyl)sulfonyl]methyl]cyclohexyl]amino]carbonyl]amino]-3,3-dimethyl-1-oxobutyl]-6,6-dimethyl-, (1R,2S,5S)- |
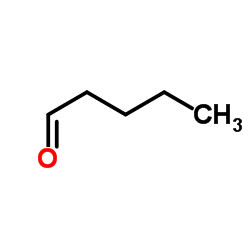 CAS#:110-62-3
CAS#:110-62-3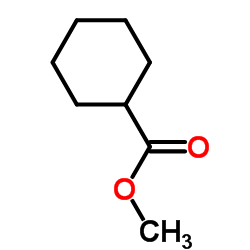 CAS#:4630-82-4
CAS#:4630-82-4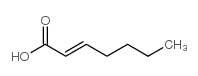 CAS#:18999-28-5
CAS#:18999-28-5 CAS#:40195-26-4
CAS#:40195-26-4 CAS#:254438-52-3
CAS#:254438-52-3 CAS#:1184928-27-5
CAS#:1184928-27-5![(1R,2S,5S)-3-[(2S)-2-[[1-(tert-butylsulfonylmethyl)cyclohexyl]carbamoylamino]-3,3-dimethylbutanoyl]-N-[(3S)-1-(cyclopropylamino)-2-hydroxy-1-oxoheptan-3-yl]-6,6-dimethyl-3-azabicyclo[3.1.0]hexane-2-carboxamide Structure](https://image.chemsrc.com/caspic/027/1208245-90-2.png) CAS#:1208245-90-2
CAS#:1208245-90-2 CAS#:108-94-1
CAS#:108-94-1