2-Methyl-2-propanyl 1-pyrrolidinecarboxylate
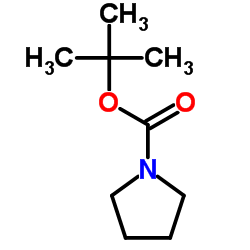
2-Methyl-2-propanyl 1-pyrrolidinecarboxylate structure
|
Common Name | 2-Methyl-2-propanyl 1-pyrrolidinecarboxylate | ||
|---|---|---|---|---|
| CAS Number | 86953-79-9 | Molecular Weight | 171.237 | |
| Density | 1.0±0.1 g/cm3 | Boiling Point | 221.4±9.0 °C at 760 mmHg | |
| Molecular Formula | C9H17NO2 | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | 87.7±18.7 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
| Name | 1-Boc-Pyrrolidine |
|---|---|
| Synonym | More Synonyms |
| Density | 1.0±0.1 g/cm3 |
|---|---|
| Boiling Point | 221.4±9.0 °C at 760 mmHg |
| Molecular Formula | C9H17NO2 |
| Molecular Weight | 171.237 |
| Flash Point | 87.7±18.7 °C |
| Exact Mass | 171.125931 |
| PSA | 29.54000 |
| LogP | 1.49 |
| Vapour Pressure | 0.1±0.4 mmHg at 25°C |
| Index of Refraction | 1.473 |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H319-H335 |
| Precautionary Statements | P261-P305 + P351 + P338 |
| Personal Protective Equipment | Eyeshields;full-face respirator (US);Gloves;multi-purpose combination respirator cartridge (US);type ABEK (EN14387) respirator filter |
| Hazard Codes | Xi:Irritant; |
| Risk Phrases | R36/37/38 |
| Safety Phrases | S26-S36 |
| RIDADR | NA 1993 / PGIII |
| WGK Germany | 3 |
| HS Code | 2933990090 |
| Precursor 10 | |
|---|---|
| DownStream 10 | |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Enantioselective, palladium-catalyzed α-arylation of N-Boc pyrrolidine: in situ react IR spectroscopic monitoring, scope, and synthetic applications.
J. Org. Chem. 76(15) , 5936-53, (2011) A comprehensive study of the enantioselective Pd-catalyzed α-arylation of N-Boc pyrrolidine has been carried out. The protocol involves deprotonation of N-Boc pyrrolidine using s-BuLi/(-)-sparteine in... |
|
|
Asymmetric synthesis of enantioenriched (+)-elaeokanine A.
J. Org. Chem. 71(15) , 5674-8, (2006) The key transformation in the total synthesis of (+)-elaeokanine A was accomplished by asymmetric deprotonation of N-Boc pyrrolidine, followed by the reaction of the in situ generated enantioenriched ... |
|
|
Enantioselective, palladium-catalyzed α-arylation of N-boc-pyrrolidine. Campos KR, et al.
J. Am. Chem. Soc. 128(11) , 3538-3539, (2006)
|
| tert-butyl pyrrolidine-1-carboxylate |
| MFCD00216581 |
 CAS#:123-75-1
CAS#:123-75-1 CAS#:24424-99-5
CAS#:24424-99-5 CAS#:5176-27-2
CAS#:5176-27-2 CAS#:15761-39-4
CAS#:15761-39-4 CAS#:109-96-6
CAS#:109-96-6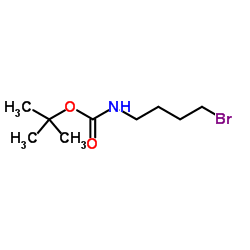 CAS#:164365-88-2
CAS#:164365-88-2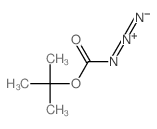 CAS#:1070-19-5
CAS#:1070-19-5 CAS#:109-97-7
CAS#:109-97-7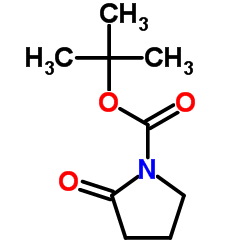 CAS#:85909-08-6
CAS#:85909-08-6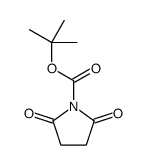 CAS#:41839-96-7
CAS#:41839-96-7 CAS#:57294-38-9
CAS#:57294-38-9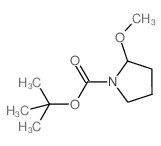 CAS#:144688-69-7
CAS#:144688-69-7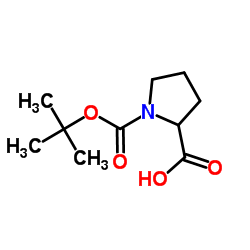 CAS#:37784-17-1
CAS#:37784-17-1![2-(2,9,9-TriMethyl-3,5-dioxa-4-bora-tricyclo[6.1.1.02,6]dec-4-yl)-pyrrolidine structure](https://image.chemsrc.com/caspic/477/205116-75-2.png) CAS#:205116-75-2
CAS#:205116-75-2 CAS#:115-11-7
CAS#:115-11-7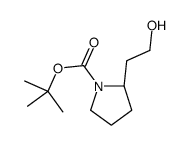 CAS#:132482-06-5
CAS#:132482-06-5 CAS#:88790-38-9
CAS#:88790-38-9
