Thiolutin
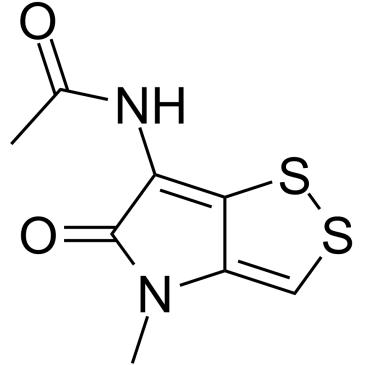
Thiolutin structure
|
Common Name | Thiolutin | ||
|---|---|---|---|---|
| CAS Number | 87-11-6 | Molecular Weight | 228.291 | |
| Density | 1.6±0.1 g/cm3 | Boiling Point | 478.6±45.0 °C at 760 mmHg | |
| Molecular Formula | C8H8N2O2S2 | Melting Point | 273-276℃ | |
| MSDS | Chinese USA | Flash Point | 243.3±28.7 °C | |
| Symbol |

GHS06 |
Signal Word | Danger | |
Use of ThiolutinThiolutin (Acetopyrrothin) is a disulfide-containing antibiotic and anti-angiogenic compound produced by Streptomyces. Thiolutin inhibits the JAMM metalloproteases Csn5, Associated-molecule-with-the-SH3-Domain-of-STAM (AMSH) and Brcc36[1]. Thiolutin is a potent and selective inhibitor of endothelial cell adhesion accompanied by rapid induction of Heat-shock protein beta-1 (Hsp27) phosphorylation[2]. |
| Name | N-(4-methyl-5-oxodithiolo[4,3-b]pyrrol-6-yl)acetamide |
|---|---|
| Synonym | More Synonyms |
| Description | Thiolutin (Acetopyrrothin) is a disulfide-containing antibiotic and anti-angiogenic compound produced by Streptomyces. Thiolutin inhibits the JAMM metalloproteases Csn5, Associated-molecule-with-the-SH3-Domain-of-STAM (AMSH) and Brcc36[1]. Thiolutin is a potent and selective inhibitor of endothelial cell adhesion accompanied by rapid induction of Heat-shock protein beta-1 (Hsp27) phosphorylation[2]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.6±0.1 g/cm3 |
|---|---|
| Boiling Point | 478.6±45.0 °C at 760 mmHg |
| Melting Point | 273-276℃ |
| Molecular Formula | C8H8N2O2S2 |
| Molecular Weight | 228.291 |
| Flash Point | 243.3±28.7 °C |
| Exact Mass | 228.002716 |
| PSA | 107.58000 |
| LogP | 0.99 |
| Vapour Pressure | 0.0±1.2 mmHg at 25°C |
| Index of Refraction | 1.721 |
| Water Solubility | DMSO: 5 mg/mL |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Symbol |

GHS06 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H300 |
| Precautionary Statements | P264-P301 + P310 |
| Personal Protective Equipment | Eyeshields;Faceshields;full-face particle respirator type N100 (US);Gloves;respirator cartridge type N100 (US);type P1 (EN143) respirator filter;type P3 (EN 143) respirator cartridges |
| Hazard Codes | T+: Very toxic;T: Toxic; |
| Risk Phrases | 28 |
| Safety Phrases | S45 |
| RIDADR | UN 2811 6.1/PG 2 |
| WGK Germany | 3 |
| RTECS | JP1355000 |
| HS Code | 2933990090 |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Condensin targets and reduces unwound DNA structures associated with transcription in mitotic chromosome condensation.
Nat. Commun. 6 , 7815, (2015) Chromosome condensation is a hallmark of mitosis in eukaryotes and is a prerequisite for faithful segregation of genetic material to daughter cells. Here we show that condensin, which is essential for... |
|
|
Thiophene-degrading Escherichia coli mutants possess sulfone oxidase activity and show altered resistance to sulfur-containing antibiotics.
Appl. Environ. Microbiol. 56(10) , 3179-85, (1990) We have previously isolated mutants of Escherichia coli which show increased oxidation of heterocyclic furan and thiophene substrates. We have now found that strains carrying the thdA mutation express... |
|
|
Mapping of two transcription mutations (tlnI and tlnII) conferring thiolutin resistance, adjacent to dnaZ and rho in Escherichia coli.
Mol. Gen. Genet. 180(3) , 609-15, (1980) Two mutations in Escherichia coli conferring resistance to the transcription initiation inhibitor, thiolutin, have been mapped. One of these mutations (tln-I)( maps at 10.2 min on the genetic map and ... |
| acetopyrrothine |
| N-(4-Methyl-5-oxo-4,5-dihydro[1,2]dithiolo[4,3-b]pyrrol-6-yl)acetamide |
| N-(4-methyl-5-oxo-4,5-dihydro-[1,2]dithiolo[4,3-b]pyrrol-6-yl)-acetamide |
| 6-(Acetylamino)-4-methyl-1,2-dithiolo(4,3-b)pyrrol-5(4H)-one |
| 6-Acetamido-4-methyl-1,2-dithiolo<4,3-b>pyrrol-5(4H)-on |
| Acetopyrrothin |
| 6-Acetamido-4-methyl-1,2-dithiolo(4,3-b)pyrrol-5(4H)-one |
| 6-(Acetamido)-4-methyl-1,2-dithiolo[4,3-b]pyrrol-5(4H)-one |
| MFCD07370147 |
| 3-Acetamido-5-methylpyrrolin-4-one[4,3-d]-1,2-dithiole |
| Acetamide, N-(4,5-dihydro-4-methyl-5-oxo-1,2-dithiolo[4,3-b]pyrrol-6-yl)- |
| Thiolutin |
| N-(4-Methyl-5-oxo-4,5-dihydro-[1,2]dithiolo[4,3-b]pyrrol-6-yl)-acetamid |
| 3-Acetamido-5-methylpyrrolin-4-one(4,3-d)-1,2-dithiole |
| 6-acetylamino-4-methyl-4H-[1,2]dithiolo[4,3-b]pyrrol-5-one |
![Methyl-6-acetamido-4,5-dihydro-4-methyl-5-oxo-1,2-dithiolo[4,3-b]pyrrol-3-carboxylat Structure](https://image.chemsrc.com/caspic/122/139102-08-2.png) CAS#:139102-08-2
CAS#:139102-08-2![N-(5,6-Dihydro-5-methyl-6-oxo-2,2-diphenyl-1,3-dithiino[5,4-b]pyrrol-7-yl)acetamid Structure](https://image.chemsrc.com/caspic/373/142705-66-6.png) CAS#:142705-66-6
CAS#:142705-66-6![Methyl-4,5-dihydro-4-methyl-5-oxo-1,2-dithiolo[4,3-b]pyrrol-3-carboxylat Structure](https://image.chemsrc.com/caspic/171/139101-74-9.png) CAS#:139101-74-9
CAS#:139101-74-9![5-Methyl-2,2-diphenyl-1,3-dithiino[5,4-b]pyrrol-6(5H)-on Structure](https://image.chemsrc.com/caspic/171/139101-96-5.png) CAS#:139101-96-5
CAS#:139101-96-5![7-Acetyl-5-methyl-2,2-diphenyl-1,3-dithiino[5,4-b]pyrrol-6(5H)-on Structure](https://image.chemsrc.com/caspic/095/139101-98-7.png) CAS#:139101-98-7
CAS#:139101-98-7![7-(1-Hydroxyiminoethyl)-5-methyl-2,2-diphenyl-1,3-dithiino[5,4-b]pyrrol-6(5H)-on Structure](https://image.chemsrc.com/caspic/436/139102-00-4.png) CAS#:139102-00-4
CAS#:139102-00-4![5,6,7,7a-Tetrahydro-7-hydroxy-5-methyl-6-oxo-2,2-diphenyl-1,3-dithiino[5,4-b]pyrrol-7-carbonsaeure Structure](https://image.chemsrc.com/caspic/078/139101-81-8.png) CAS#:139101-81-8
CAS#:139101-81-8
