E 2012
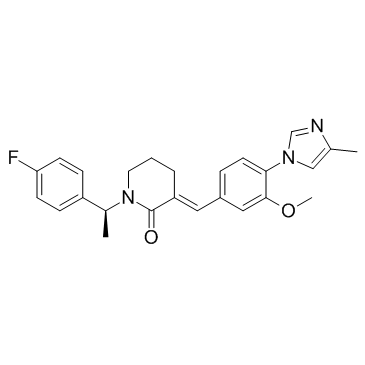
E 2012 structure
|
Common Name | E 2012 | ||
|---|---|---|---|---|
| CAS Number | 870843-42-8 | Molecular Weight | 419.49100 | |
| Density | 1.195g/cm3 | Boiling Point | 649.168ºC at 760 mmHg | |
| Molecular Formula | C25H26FN3O2 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | 346.405ºC | |
Use of E 2012E 2012 is a potent γ-secretase modulator.IC50 value:Target: γ-secretaseIn the present study, 9 dogs were treated with a single dose of the γ-secretase modulator E2012, the γ-secretase inhibitor LY450139, or vehicle with a dosing interval of 1 week. The isoform Aβ(1-37) was significantly increased in a dose-dependent manner in response to treatment with E2012, while Aβ(1-39), Aβ(1-40) and A(1-42) decreased [1].E2012, a gamma secretase modulator without affecting Notch processing, aimed at Alzheimer's disease by reduction of amyloid β-42, induced cataract following repeated doses in the rat.E2012 inhibits 3β-hydroxysterol Δ24-reductase (DHCR24) at the final step in the cholesterol biosynthesis. In vivo lenticular concentration of E2012 after 13-week repeated dose with cataract was well above those where inhibition was observed in vitro.E2012 induces cataract in the rat by inhibiting DHCR24 at the final step of cholesterol synthesis with associated elevation in desmosterol within the lens, preceded by desmosterol changes that would serve as a predictive safety biomarker for lenticular opacity [2]. |
| Name | (3E)-1-[(1S)-1-(4-fluorophenyl)ethyl]-3-[[3-methoxy-4-(4-methylimidazol-1-yl)phenyl]methylidene]piperidin-2-one |
|---|---|
| Synonym | More Synonyms |
| Description | E 2012 is a potent γ-secretase modulator.IC50 value:Target: γ-secretaseIn the present study, 9 dogs were treated with a single dose of the γ-secretase modulator E2012, the γ-secretase inhibitor LY450139, or vehicle with a dosing interval of 1 week. The isoform Aβ(1-37) was significantly increased in a dose-dependent manner in response to treatment with E2012, while Aβ(1-39), Aβ(1-40) and A(1-42) decreased [1].E2012, a gamma secretase modulator without affecting Notch processing, aimed at Alzheimer's disease by reduction of amyloid β-42, induced cataract following repeated doses in the rat.E2012 inhibits 3β-hydroxysterol Δ24-reductase (DHCR24) at the final step in the cholesterol biosynthesis. In vivo lenticular concentration of E2012 after 13-week repeated dose with cataract was well above those where inhibition was observed in vitro.E2012 induces cataract in the rat by inhibiting DHCR24 at the final step of cholesterol synthesis with associated elevation in desmosterol within the lens, preceded by desmosterol changes that would serve as a predictive safety biomarker for lenticular opacity [2]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.195g/cm3 |
|---|---|
| Boiling Point | 649.168ºC at 760 mmHg |
| Molecular Formula | C25H26FN3O2 |
| Molecular Weight | 419.49100 |
| Flash Point | 346.405ºC |
| Exact Mass | 419.20100 |
| PSA | 47.36000 |
| LogP | 5.03330 |
| Index of Refraction | 1.6 |
|
~88% 
E 2012 CAS#:870843-42-8 |
| Literature: Eisai RandD Management Co., Ltd. Patent: EP1950211 A1, 2008 ; Location in patent: Page/Page column 64 ; |
|
~% 
E 2012 CAS#:870843-42-8 |
| Literature: EP1953151 A1, ; Page/Page column 13 ; |
|
~82% 
E 2012 CAS#:870843-42-8 |
| Literature: Eisai RandD Management Co., Ltd. Patent: EP1950211 A1, 2008 ; Location in patent: Page/Page column 62 ; |
|
~88% 
E 2012 CAS#:870843-42-8 |
| Literature: Eisai RandD Management Co., Ltd. Patent: US2008/306272 A1, 2008 ; Location in patent: Page/Page column 8; 9 ; |
|
~58% 
E 2012 CAS#:870843-42-8 |
| Literature: Eisai RandD Management Co., Ltd. Patent: EP1950211 A1, 2008 ; Location in patent: Page/Page column 60 ; |
|
~% 
E 2012 CAS#:870843-42-8 |
| Literature: EP1950211 A1, ; Page/Page column 52-53 ; |
|
~% 
E 2012 CAS#:870843-42-8 |
| Literature: EP1950211 A1, ; Page/Page column 56-57 ; |
|
~% 
E 2012 CAS#:870843-42-8 |
| Literature: EP1950211 A1, ; Page/Page column 58-59 ; |
|
~76% 
E 2012 CAS#:870843-42-8 |
| Literature: Eisai RandD Management Co., Ltd. Patent: EP1950211 A1, 2008 ; Location in patent: Page/Page column 64 ; |
| Precursor 3 | |
|---|---|
| DownStream 0 | |
| (S,E)-1-(1-(4-fluorophenyl)ethyl)-3-(3-methoxy-4-(4-methyl-1H-imidazol-1-yl)benzylidene)piperidin-2-one |
| (3E)-1-[(1S)-1-(4-fluorophenyl)ethyl]-3-[3-methoxy-4-(4-methyl-1H-imidazol-1-yl)benzylidene]piperidin-2-one |
| E-2012 |
| (e)-1-((1s)-1-(4-fluorophenyl)ethyl)-3-(3-methoxy-4-(4-methyl-1h-imidazol-1-yl)benzylidene)piperidin-2-one |
| cc-582 |
| E 2012 |
![acetic acid {1-[(1S)-1-(4-fluorophenyl)ethyl]-2-oxopiperidin-3-yl}[3-methoxy-4-(4-methyl-1H-imidazol-1-yl)phenyl]methyl ester structure](https://image.chemsrc.com/caspic/444/937026-29-4.png)
![(3E)-5-chloro-2-[3-methoxy-4-(4-methyl-1H-imidazol-1-yl)benzylidene]valeric acid [(S)-1-(4-fluorophenyl)ethyl]amide structure](https://image.chemsrc.com/caspic/362/870843-43-9.png)

![2-Piperidinone, 1-[(1S)-1-(4-fluorophenyl)ethyl]- structure](https://image.chemsrc.com/caspic/465/937026-11-4.png)