Ramipril

Ramipril structure
|
Common Name | Ramipril | ||
|---|---|---|---|---|
| CAS Number | 87333-19-5 | Molecular Weight | 416.511 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | 616.2±55.0 °C at 760 mmHg | |
| Molecular Formula | C23H32N2O5 | Melting Point | 106-108°C | |
| MSDS | Chinese USA | Flash Point | 326.4±31.5 °C | |
Use of RamiprilRamipril is an angiotensin-converting enzyme (ACE) inhibitor with IC50 of 5 nM.Target: ACERamipril is an angiotensin-converting enzyme (ACE) inhibitor with IC50 of 5 nM [1]. Ramipril enhances the activity of ACE-associated CK2 and the phosphorylation of ACE Ser1270 in cultured endothelial cells, but is unable to activate JNK or stimulate the nuclear accumulation of c-Jun in endothelial cells expressing a S1270A ACE mutant or in ACE-deficient cells. Prolonged Ramipril treatment increases ACE expression in primary cultures of human endothelial cells and in vivo (mouse lung), which can be prevented by pretreatment with the JNK inhibitor SP600125 [2].Chronic in vivo administration of Ramipril to rats at a dosage that has similar hypotensive effects in vitro HUVECs significantly reduces the rate of LPS-induced apoptosis compared to the other ACE inhibitors, which contrasts with the apoptosis effect in vitro [3]. Ramipril inhibits systolic blood pressure (SBP) with IC50 of 1.97 mg/kg in spontaneously hypertensive rats (SHR). When in combination with AT1-receptor blockade by candesartan-cilexetil increases SBP reduction synergistically rather than additively [4]. |
| Name | ramipril |
|---|---|
| Synonym | More Synonyms |
| Description | Ramipril is an angiotensin-converting enzyme (ACE) inhibitor with IC50 of 5 nM.Target: ACERamipril is an angiotensin-converting enzyme (ACE) inhibitor with IC50 of 5 nM [1]. Ramipril enhances the activity of ACE-associated CK2 and the phosphorylation of ACE Ser1270 in cultured endothelial cells, but is unable to activate JNK or stimulate the nuclear accumulation of c-Jun in endothelial cells expressing a S1270A ACE mutant or in ACE-deficient cells. Prolonged Ramipril treatment increases ACE expression in primary cultures of human endothelial cells and in vivo (mouse lung), which can be prevented by pretreatment with the JNK inhibitor SP600125 [2].Chronic in vivo administration of Ramipril to rats at a dosage that has similar hypotensive effects in vitro HUVECs significantly reduces the rate of LPS-induced apoptosis compared to the other ACE inhibitors, which contrasts with the apoptosis effect in vitro [3]. Ramipril inhibits systolic blood pressure (SBP) with IC50 of 1.97 mg/kg in spontaneously hypertensive rats (SHR). When in combination with AT1-receptor blockade by candesartan-cilexetil increases SBP reduction synergistically rather than additively [4]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 616.2±55.0 °C at 760 mmHg |
| Melting Point | 106-108°C |
| Molecular Formula | C23H32N2O5 |
| Molecular Weight | 416.511 |
| Flash Point | 326.4±31.5 °C |
| Exact Mass | 416.231110 |
| PSA | 95.94000 |
| LogP | 3.41 |
| Vapour Pressure | 0.0±1.9 mmHg at 25°C |
| Index of Refraction | 1.556 |
| Storage condition | 2-8°C |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Hazard Codes | Xi |
| Risk Phrases | R36/38 |
| Safety Phrases | S26-S37/39 |
| RIDADR | NONH for all modes of transport |
| RTECS | GY5879600 |
| HS Code | 3004909090 |
|
~95% 
Ramipril CAS#:87333-19-5 |
| Literature: Malakondaiah, Golla China; Gurav; Reddy, Lekkala Amarnath; Babu, Karrothu Srihari; Bhaskar, Bolugoddu Vijaya; Reddy, Padi Pratap; Bhattacharya, Apurba; Anand, Ramasamy Vijaya Synthetic Communications, 2008 , vol. 38, # 11 p. 1737 - 1744 |
|
~89% 
Ramipril CAS#:87333-19-5 |
| Literature: DSM IP ASSETS B.V. Patent: WO2009/62999 A1, 2009 ; Location in patent: Page/Page column 8 ; |
|
~% 
Ramipril CAS#:87333-19-5 |
| Literature: WO2005/108366 A1, ; Page/Page column 12 ; |
|
~% 
Ramipril CAS#:87333-19-5 |
| Literature: Synthetic Communications, , vol. 35, # 2 p. 243 - 248 |
|
~% 
Ramipril CAS#:87333-19-5 |
| Literature: Synthetic Communications, , vol. 35, # 2 p. 243 - 248 |
|
~% 
Ramipril CAS#:87333-19-5 |
| Literature: Arzneimittel-Forschung/Drug Research, , vol. 34, # 10 B p. 1399 - 1401 |
|
~% 
Ramipril CAS#:87333-19-5 |
| Literature: Arzneimittel-Forschung/Drug Research, , vol. 34, # 10 B p. 1399 - 1401 |
|
~% 
Ramipril CAS#:87333-19-5 |
| Literature: Arzneimittel-Forschung/Drug Research, , vol. 34, # 10 B p. 1399 - 1401 |
|
~% 
Ramipril CAS#:87333-19-5 |
| Literature: Arzneimittel-Forschung/Drug Research, , vol. 34, # 10 B p. 1399 - 1401 |
| Precursor 9 | |
|---|---|
| DownStream 1 | |
| HS Code | 3004909090 |
|---|
|
Clopidogrel bioactivation and risk of bleeding in patients cotreated with angiotensin-converting enzyme inhibitors after myocardial infarction: a proof-of-concept study.
Clin. Pharmacol. Ther. 96(6) , 713-22, (2014) Clopidogrel is an oral antiplatelet prodrug, the majority of which is hydrolyzed to an inactive metabolite by hepatic carboxylesterase 1 (CES1). Most angiotensin-converting enzyme inhibitors (ACEIs) a... |
|
|
[Telangiectasia during amlodipine therapy].
Ann. Dermatol. Venereol. 140(3) , 202-5, (2013) Calcium inhibitors are recommended as first-line treatment in hypertension. We report the development of telangiectasia on the trunk and upper limbs in a female patient on amlodipine (Amlor(®)) that s... |
|
|
Ramipril and hydrochlorothiazide treatment of hypertensive urgency in the ED.
Am. J. Emerg. Med. 31(10) , 1533-4, (2013)
|
| RAMACE |
| UNIPRIL |
| PRAMACE |
| vesdil |
| DELIX |
| (2S,3aS,6aS)-1-{2-[(1-ethoxy-1-oxo-4-phenylbutan-2-yl)amino]propanoyl}octahydrocyclopenta[b]pyrrole-2-carboxylic acid (non-preferred name) |
| N-(1S-Carboethoxy-3-phenylpropyl)-S-alanyl-cis,endo-2-azabicyclo[3.3.0]octane-3S-carboxylic Acid |
| Ramipril |
| Trimce |
| (2S,3aS,6aS)-1-[(2S)-2-{[(1S)-1-(ethoxycarbonyl)-3-phenylpropyl]amino}propanoyl]octahydrocyclopenta[b]pyrrole-2-carboxylic acid |
| (2S,3aS,6aS)-1-[(2S)-2-{[(2S)-1-ethoxy-1-oxo-4-phenylbutan-2-yl]amino}propanoyl]octahydrocyclopenta[b]pyrrole-2-carboxylic acid (non-preferred name) |
| [2S-[1[R*(R*)],2a,3ab,6ab]]-1-[2-[[1-(Ethoxycarbonyl)-3-phenylpropyl]amino]-1-oxopropyl]octahydrocyclopenta[b]pyrrole-2-carboxylic Acid |
| HOE-498 |
| (2S,3aS,6aS)-1-[(2S)-2-{[(2S)-1-Ethoxy-1-oxo-4-phenyl-2-butanyl]amino}propanoyl]octahydrocyclopenta[b]pyrrole-2-carboxylic acid |
| (2S,3aS,6aS)-1-[(S)-N-[(S)-1-Carboxy-3-phenylpropyl]alanyl]octahydrocyclopenta[b]pyrrole-2-carboxylic Acid 1-Ethyl Ester |
| MFCD00865775 |
| (2S,3aS,6aS)-1-[(2S)-2-{[(1S)-1-(ethoxycarbonyl)-3-phenylpropyl]amino}propanoyl]octahydrocyclopenta[b]pyrrole-2-carboxylic acid (non-preferred name) |
| ALTACE |
| CARDACE |
| TRITACE |
![2-[N-[(S)-1-ETHOXYCARBONYL-3-PHENYLPROPYL]-L-ALANYL]-(1S,3S,5S)-2-AZABICYCLO[3.3.0]OCTANE-3-CARBOXYLIC ACID, BENZYL ESTER structure](https://image.chemsrc.com/caspic/113/87269-88-3.png)
![N-[(S)-1-carbethoxy-3-phenyl-propyl]-S-alaninoyl chloride hydrochloride structure](https://image.chemsrc.com/caspic/294/114192-42-6.png)
![3-Azabicyclo[3.3.0]octane-2-carboxylic acid structure](https://image.chemsrc.com/caspic/473/109428-53-7.png)
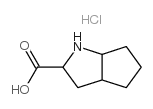
![Ethyl (S)-2-[(S)-4-methyl-2,5-dioxo-1,3-oxazolidin-3-yl]-4-phenylbutyrate structure](https://image.chemsrc.com/caspic/045/84793-24-8.png)
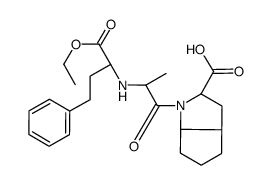
![N-[(S)-1-Ethoxycarbonyl-3-phenylpropyl]-L-alanine structure](https://image.chemsrc.com/caspic/376/82717-96-2.png)
![N-[1(S)-ethoxycarbonyl-3-phenylpropyl]-(S)-alanine-2'-benzothiazolylthio ester structure](https://image.chemsrc.com/caspic/494/124492-03-1.png)
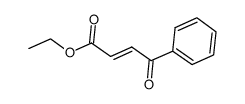
![(1S,3S,5S)-2-Azabicyclo[3,3,0]octane-3-carborylic acid benzyl ester hydrochloride structure](https://image.chemsrc.com/caspic/151/87269-87-2.png)

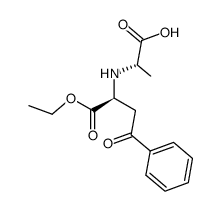
 CAS#:87269-97-4
CAS#:87269-97-4