Quinaldine
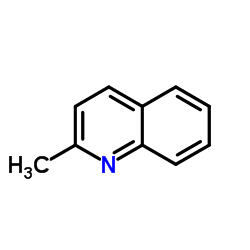
Quinaldine structure
|
Common Name | Quinaldine | ||
|---|---|---|---|---|
| CAS Number | 91-63-4 | Molecular Weight | 143.185 | |
| Density | 1.1±0.1 g/cm3 | Boiling Point | 246.5±0.0 °C at 760 mmHg | |
| Molecular Formula | C10H9N | Melting Point | -2 °C | |
| MSDS | Chinese USA | Flash Point | 79.4±0.0 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
| Purity | Quantity | Budget | Inquiry |
|---|
| Name | 2-Methylquinoline |
|---|---|
| Synonym | More Synonyms |
| Density | 1.1±0.1 g/cm3 |
|---|---|
| Boiling Point | 246.5±0.0 °C at 760 mmHg |
| Melting Point | -2 °C |
| Molecular Formula | C10H9N |
| Molecular Weight | 143.185 |
| Flash Point | 79.4±0.0 °C |
| Exact Mass | 143.073502 |
| PSA | 12.89000 |
| LogP | 2.54 |
| Vapour Pressure | 0.0±0.4 mmHg at 25°C |
| Index of Refraction | 1.625 |
| Stability | Stable, but may be light sensitive. Combustible. Incompatible with strong oxidizing agents. |
| Water Solubility | PRACTICALLY INSOLUBLE |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302 + H312 |
| Precautionary Statements | P280 |
| Personal Protective Equipment | Eyeshields;Faceshields;full-face respirator (US);Gloves;multi-purpose combination respirator cartridge (US);type ABEK (EN14387) respirator filter |
| Hazard Codes | Xn:Harmful |
| Risk Phrases | R21/22;R36/37/38;R68 |
| Safety Phrases | S36-S45-S36/37/39-S26 |
| RIDADR | 2810.0 |
| WGK Germany | 3 |
| RTECS | UZ9625000 |
| Hazard Class | 6.1 |
| HS Code | 29334990 |
~95% |
| Literature: Florida State University Patent: EP1193252 A2, 2002 ; Location in patent: Page 16 ; |
~90% |
| Literature: Florida State University Patent: EP1193252 A2, 2002 ; Location in patent: Page 15 ; |
~79% |
| Literature: Gennari, Cesare; Carcano, Michela; Donghi, Monica; Mongelli, Nicola; Vanotti, Ermes; Vulpetti, Anna Journal of Organic Chemistry, 1997 , vol. 62, # 14 p. 4746 - 4755 |
| HS Code | 2933499090 |
|---|---|
| Summary | 2933499090. other compounds containing in the structure a quinoline or isoquinoline ring-system (whether or not hydrogenated), not further fused. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
The PaaX-type repressor MeqR2 of Arthrobacter sp. strain Rue61a, involved in the regulation of quinaldine catabolism, binds to its own promoter and to catabolic promoters and specifically responds to anthraniloyl coenzyme A.
J. Bacteriol. 195(5) , 1068-80, (2013) The genes coding for quinaldine catabolism in Arthrobacter sp. strain Rue61a are clustered on the linear plasmid pAL1 in two upper pathway operons (meqABC and meqDEF) coding for quinaldine conversion ... |
|
|
Quantitative structure-activity relationship analysis of inhibitors of the nicotine metabolizing CYP2A6 enzyme.
J. Med. Chem. 48 , 440-9, (2005) The purpose of this study was to develop screening and in silico modeling methods to obtain accurate information on the active center of CYP2A6, a nicotine oxidizing enzyme. The inhibitory potencies o... |
|
|
Exploring QSAR and QAAR for inhibitors of cytochrome P450 2A6 and 2A5 enzymes using GFA and G/PLS techniques
Eur. J. Med. Chem. 44 , 1941-51, (2009) A series of naphthalene and non-naphthalene derivatives ( n = 42) having cytochrome P450 2A6 and 2A5 inhibitory activities, reported by Rahnasto et al., were subjected to QSAR and QAAR studies. The an... |
| Quinoline, 2-methyl- |
| Quinaldine |
| MFCD00006756 |
| EINECS 202-085-1 |
| 2-Methylquinoline |
| Fasudil Impurity 10 |
![1-hydroxy-7β-triethylsilyloxy-9-oxo-10β-acetyloxy-5β,20-epoxytax-11-ene-2α,4,13α-triyl 4-acetate 2-benzoate 13-[(2R,3S)-3-benzoylamino-2-triethylsilyloxy-3-phenylpropanoate] structure](https://image.chemsrc.com/caspic/484/135365-62-7.png)
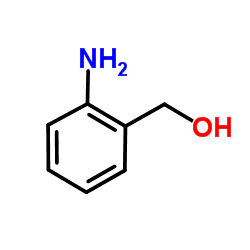 CAS#:5344-90-1
CAS#:5344-90-1 CAS#:67-64-1
CAS#:67-64-1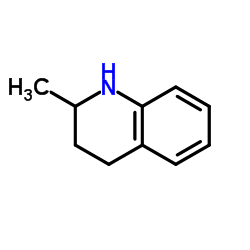 CAS#:1780-19-4
CAS#:1780-19-4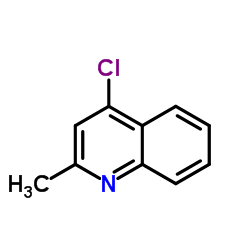 CAS#:4295-06-1
CAS#:4295-06-1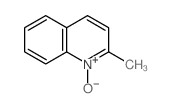 CAS#:1076-28-4
CAS#:1076-28-4 CAS#:552-89-6
CAS#:552-89-6 CAS#:64-17-5
CAS#:64-17-5 CAS#:201230-82-2
CAS#:201230-82-2 CAS#:54408-52-5
CAS#:54408-52-5 CAS#:108165-95-3
CAS#:108165-95-3 CAS#:73348-49-9
CAS#:73348-49-9 CAS#:75-07-0
CAS#:75-07-0 CAS#:5371-52-8
CAS#:5371-52-8 CAS#:1198-37-4
CAS#:1198-37-4 CAS#:104293-35-8
CAS#:104293-35-8 CAS#:37597-46-9
CAS#:37597-46-9 CAS#:108-93-0
CAS#:108-93-0
