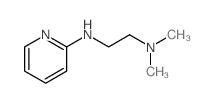
Tripelennamine
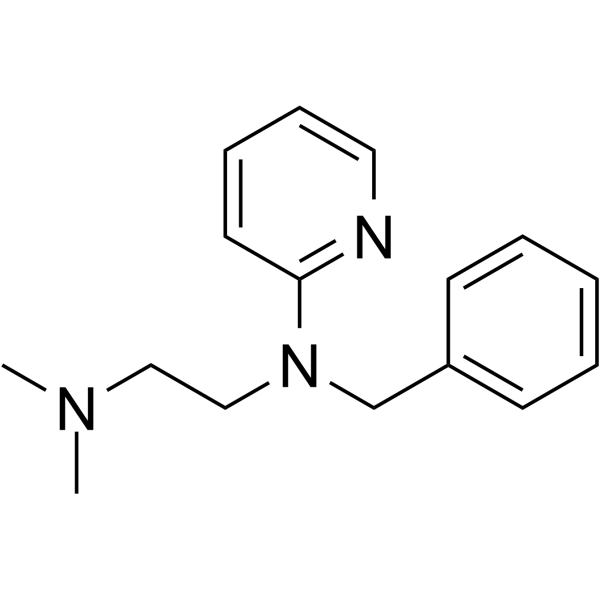
Tripelennamine structure
|
Common Name | Tripelennamine | ||
|---|---|---|---|---|
| CAS Number | 91-81-6 | Molecular Weight | 255.35800 | |
| Density | 1.0683 (rough estimate) | Boiling Point | 185 - 190ºC at 1.7 mm Hg | |
| Molecular Formula | C16H21N3 | Melting Point | 25°C | |
| MSDS | N/A | Flash Point | N/A | |
Use of TripelennamineTripelennamine, an ethylenediamine derivative, is a potent histamine H1-receptor antagonist. Tripelennamine lessens the allergic response of the organism caused by histamine. Tripelennamine can be used for the research of rhinitis, conjunctivitis, and allergic and anaphylactic reactions[1][2][3]. |
| Name | N'-benzyl-N,N-dimethyl-N'-pyridin-2-ylethane-1,2-diamine |
|---|---|
| Synonym | More Synonyms |
| Description | Tripelennamine, an ethylenediamine derivative, is a potent histamine H1-receptor antagonist. Tripelennamine lessens the allergic response of the organism caused by histamine. Tripelennamine can be used for the research of rhinitis, conjunctivitis, and allergic and anaphylactic reactions[1][2][3]. |
|---|---|
| Related Catalog | |
| Target |
H1 Receptor |
| References |
| Density | 1.0683 (rough estimate) |
|---|---|
| Boiling Point | 185 - 190ºC at 1.7 mm Hg |
| Melting Point | 25°C |
| Molecular Formula | C16H21N3 |
| Molecular Weight | 255.35800 |
| Exact Mass | 255.17400 |
| PSA | 19.37000 |
| LogP | 2.64980 |
| Index of Refraction | nD25 1.5759-1.5765 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
|
~75% 
Tripelennamine CAS#:91-81-6 |
| Literature: Hamid, M. Haniti S. A.; Allen, C. Liana; Lamb, Gareth W.; Maxwell, Aoife C.; Maytum, Hannah C.; et al. Journal of the American Chemical Society, 2009 , vol. 131, p. 1766 - 1774 |
|
~% 
Tripelennamine CAS#:91-81-6 |
| Literature: Gogate, Priyanka N.; Ethirajan, Manivannan; Kurenova, Elena V.; Magis, Andrew T.; Pandey, Ravindra K.; Cance, William G. European Journal of Medicinal Chemistry, 2014 , vol. 80, p. 154 - 156 |
|
~% 
Tripelennamine CAS#:91-81-6 |
| Literature: Gogate, Priyanka N.; Ethirajan, Manivannan; Kurenova, Elena V.; Magis, Andrew T.; Pandey, Ravindra K.; Cance, William G. European Journal of Medicinal Chemistry, 2014 , vol. 80, p. 154 - 156 |
| Piribenzil |
| Tonaril |
| N-benzyl-N',N'-dimethyl-N-pyridin-2-yl-ethane-1,2-diamine |
| Benzoxale |
| Tripelennamin |
| Pyribenzamine |
| Cizaron |
| N-benzyl-N',N'-dimethyl-N-[2]pyridyl-ethylenediamine |
| Resistamine |
| tripelenamin |
| Tripelennamina |
| tripelennamine |
| Pyrinamine base |