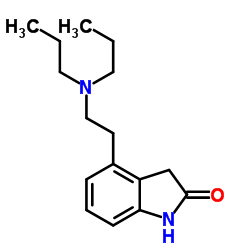
Ropinirole hydrochloride
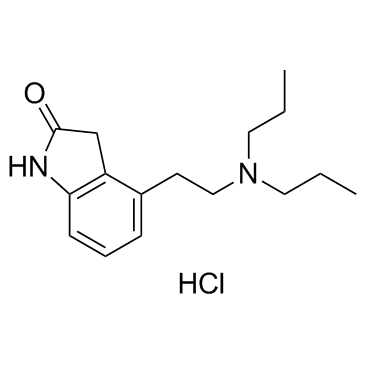
Ropinirole hydrochloride structure
|
Common Name | Ropinirole hydrochloride | ||
|---|---|---|---|---|
| CAS Number | 91374-20-8 | Molecular Weight | 296.836 | |
| Density | N/A | Boiling Point | 410.5ºC at 760mmHg | |
| Molecular Formula | C16H25ClN2O | Melting Point | 241-243ºC | |
| MSDS | Chinese USA | Flash Point | N/A | |
| Symbol |


GHS07, GHS09 |
Signal Word | Warning | |
Use of Ropinirole hydrochlorideRopinirole hydrochloride(SKF101468 hydrochloride) a selective dopamine D2 receptor inhibitor with IC50 of 29 nM.Target: Dopamine D2 ReceptorRopinirole (50 mg/kg, i.p.) causes biphasic spontaneous locomotor activity in mice. Ropinirole (0.05-1.0 mg/kg SC) dose-dependently inhibits the dyskinesias induced by 2-di-n-propylamino-5,6-di-hydroxytetralin in mice. Ropirtirole, at doses of 1 and 10 μg, injected unilaterally directly into the striatum of the rat causes marked, contralateral (away from the side of injection) asymmetry and circling in mice. Ropinirole (0.05-1.0 mg/kg SC or 0.1 mg/kg PO) reverses all motor and behavioural deficits induced by MPTP in marmosets [1]. Ropinirole (2 mg/kg, i.p.) for 7 days increases GSH, catalase and SOD activities in the striatum and protected striatal dopaminergic neurons against 6-hydroxydopamine (6-OHDA) in mice [2]. Ropinirole (0.2 mg/kg, i.p.) improves the use of previously akinetic forelimb and produced robust circling behavior in lesioned rats with striatal over-expression of both D2R and D3R compared to lesioned animals that received blank vector. The subtherapeutic dose of ropinirole generates only modest motor effects in lesioned rats with sole over-expression of D2R or D3R [3]. Ropinirole (1-8 mg t.i.d.) is rapidly and completely absorbed with oral bioavailability of 55%, clearance of 780 mL/min, elimination half-life of 6 hours in healthy volunteer. Since the major route of elimination for Ropinirole is by the CYP enzyme system, mainly by CYP1A2 and also by CYP3A4, inhibition of the former and possibly the latter may reduce the agent's clearance and lead to drug accumulation [4]. |
| Name | 4-(2-(Dipropylamino)ethyl)indolin-2-one hydrochloride |
|---|---|
| Synonym | More Synonyms |
| Description | Ropinirole hydrochloride(SKF101468 hydrochloride) a selective dopamine D2 receptor inhibitor with IC50 of 29 nM.Target: Dopamine D2 ReceptorRopinirole (50 mg/kg, i.p.) causes biphasic spontaneous locomotor activity in mice. Ropinirole (0.05-1.0 mg/kg SC) dose-dependently inhibits the dyskinesias induced by 2-di-n-propylamino-5,6-di-hydroxytetralin in mice. Ropirtirole, at doses of 1 and 10 μg, injected unilaterally directly into the striatum of the rat causes marked, contralateral (away from the side of injection) asymmetry and circling in mice. Ropinirole (0.05-1.0 mg/kg SC or 0.1 mg/kg PO) reverses all motor and behavioural deficits induced by MPTP in marmosets [1]. Ropinirole (2 mg/kg, i.p.) for 7 days increases GSH, catalase and SOD activities in the striatum and protected striatal dopaminergic neurons against 6-hydroxydopamine (6-OHDA) in mice [2]. Ropinirole (0.2 mg/kg, i.p.) improves the use of previously akinetic forelimb and produced robust circling behavior in lesioned rats with striatal over-expression of both D2R and D3R compared to lesioned animals that received blank vector. The subtherapeutic dose of ropinirole generates only modest motor effects in lesioned rats with sole over-expression of D2R or D3R [3]. Ropinirole (1-8 mg t.i.d.) is rapidly and completely absorbed with oral bioavailability of 55%, clearance of 780 mL/min, elimination half-life of 6 hours in healthy volunteer. Since the major route of elimination for Ropinirole is by the CYP enzyme system, mainly by CYP1A2 and also by CYP3A4, inhibition of the former and possibly the latter may reduce the agent's clearance and lead to drug accumulation [4]. |
|---|---|
| Related Catalog | |
| References |
| Boiling Point | 410.5ºC at 760mmHg |
|---|---|
| Melting Point | 241-243ºC |
| Molecular Formula | C16H25ClN2O |
| Molecular Weight | 296.836 |
| Exact Mass | 296.165527 |
| PSA | 32.34000 |
| LogP | 3.78570 |
| Storage condition | Room temp |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |


GHS07, GHS09 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302-H400 |
| Precautionary Statements | P301 + P312 + P330 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Faceshields;Gloves |
| Hazard Codes | Xn,N |
| Risk Phrases | R22 |
| Safety Phrases | 36-60-61-37/39-26 |
| RIDADR | UN 3077 9/PG 3 |
| RTECS | NM3288250 |
| Hazard Class | 9.0 |
| HS Code | 2933790090 |
|
~89% 
Ropinirole hydr... CAS#:91374-20-8 |
| Literature: IND-SWIFT LABORATORIES LIMITED Patent: WO2008/84499 A1, 2008 ; Location in patent: Page/Page column 8 ; |
|
~50% 
Ropinirole hydr... CAS#:91374-20-8 |
| Literature: TORRENT PHARMACEUTICALS LTD Patent: WO2005/80333 A1, 2005 ; Location in patent: Page/Page column 23 ; |
|
~% 
Ropinirole hydr... CAS#:91374-20-8 |
| Literature: WO2011/30330 A2, ; Page/Page column 9 ; |
|
~% 
Ropinirole hydr... CAS#:91374-20-8 |
| Literature: WO2005/40115 A1, ; Page/Page column 6 ; |
|
~40% 
Ropinirole hydr... CAS#:91374-20-8 |
| Literature: Helm AG; CF Pharma Gyogyszergyarto Kft. Patent: EP1568689 A1, 2005 ; Location in patent: Page/Page column 7 ; |
|
~85% 
Ropinirole hydr... CAS#:91374-20-8 |
| Literature: Hayler, John D.; Howie, Simon L. B.; Giles, Robert G.; Negus, Alan; Oxley, Paul W.; Walsgrove, Timothy C.; Whiter Organic Process Research and Development, 1998 , vol. 2, # 1 p. 3 - 9 |
|
~% 
Ropinirole hydr... CAS#:91374-20-8 |
| Literature: WO2011/72704 A1, ; |
|
~% 
Ropinirole hydr... CAS#:91374-20-8 |
| Literature: Organic Process Research and Development, , vol. 2, # 1 p. 3 - 9 |
|
~% 
Ropinirole hydr... CAS#:91374-20-8 |
| Literature: Organic Process Research and Development, , vol. 2, # 1 p. 3 - 9 |
| Precursor 10 | |
|---|---|
| DownStream 1 | |
| HS Code | 2933790090 |
|---|---|
| Summary | 2933790090. other lactams. VAT:17.0%. Tax rebate rate:9.0%. . MFN tariff:9.0%. General tariff:20.0% |
|
Sustained release microspheres of ropinirole hydrochloride: effect of process parameters.
Acta. Pharm. 61 , 363-376, (2011) An emulsion solvent evaporation method was employed to prepare microspheres of ropinirole hydrochloride, a highly water soluble drug, by using ethylcellulose and PEG with the help of 32 full factorial... |
|
|
Serotonergic and dopaminergic mechanisms in graft-induced dyskinesia in a rat model of Parkinson's disease
Neurobiol. Dis. 47(3) , 393-406, (2012) Dyskinesia seen in the off-state, referred as graft-induced dyskinesia (GID), has emerged as a serious complication induced by dopamine (DA) cell transplantation in parkinsonian patients. Although the... |
|
|
Structure-activity relationship study of N⁶-(2-(4-(1H-Indol-5-yl)piperazin-1-yl)ethyl)-N⁶-propyl-4,5,6,7-tetrahydrobenzo[d]thiazole-2,6-diamine analogues: development of highly selective D3 dopamine receptor agonists along with a highly potent D2/D3 agonist and their pharmacological characterization.
J. Med. Chem. 55(12) , 5826-40, (2012) In our effort to develop multifunctional drugs against Parkinson's disease, a structure-activity-relationship study was carried out based on our hybrid molecular template targeting D2/D3 receptors. Co... |
| 2H-Indol-2-one, 4-[2-(dipropylamino)ethyl]-1,3-dihydro-, hydrochloride (1:1) |
| 4-[2-(Dipropylamino)ethyl]-1,3-dihydro-2H-indol-2-one hydrochloride |
| 4-[2-(Dipropylamino)ethyl]-1,3-dihydro-2H-indol-2-one hydrochloride (1:1) |
| 4-[2-(Dipropylamino)ethyl]-1,3-dihydro-2H-indol-2-onhydrochlorid |
| UNII-D7ZD41RZI9 |
| ropinirole hcl |
| 4-[2-(dipropylamino)éthyl]-1,3-dihydro-2H-indol-2-one chlorhydrate |
| Adartrel |
| MFCD01754173 |
| Ropinirole hydrochloride |
| 2H-indol-2-one, 4-[2-(dipropylamino)ethyl]-1,3-dihydro-, monohydrochloride |
| Requip |
| Ropinirole (hydrochloride) |

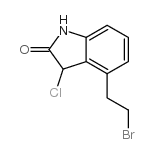
![2-{2-[2-(dipropylamino)ethyl]phenyl}-N-hydroxyacetamide structure](https://image.chemsrc.com/caspic/470/863436-07-1.png)
![4-[2'-[[(4-Methylphenyl)sulfonyl]oxy]ethyl]-1,3-dihydro-2H-indole-2-one structure](https://image.chemsrc.com/caspic/086/139122-20-6.png)
![[2-(2-methyl-3-nitro-phenyl)-ethyl]-dipropyl-amine hydrochloride structure](https://image.chemsrc.com/caspic/123/920986-68-1.png)