Refametinib (R enantiomer)
Modify Date: 2024-04-02 09:37:28
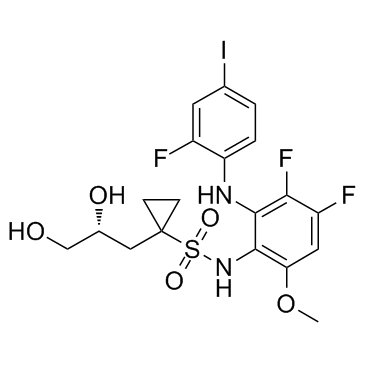
Refametinib (R enantiomer) structure
|
Common Name | Refametinib (R enantiomer) | ||
|---|---|---|---|---|
| CAS Number | 923032-38-6 | Molecular Weight | 572.337 | |
| Density | 1.8±0.1 g/cm3 | Boiling Point | 566.9±60.0 °C at 760 mmHg | |
| Molecular Formula | C19H20F3IN2O5S | Melting Point | N/A | |
| MSDS | N/A | Flash Point | 296.7±32.9 °C | |
Use of Refametinib (R enantiomer)Refametinib R enantiomer is a MEK inhibitor extracted from patent WO2007014011A2, compound 1022, has an EC50 of 2.0-15 nM. |
| Name | Cyclopropanesulfonamide, N-[3,4-difluoro-2-[(2-fluoro-4-iodophenyl)amino]-6-methoxyphenyl]-1-[(2R)-2,3-dihydroxypropyl] |
|---|---|
| Synonym | More Synonyms |
| Description | Refametinib R enantiomer is a MEK inhibitor extracted from patent WO2007014011A2, compound 1022, has an EC50 of 2.0-15 nM. |
|---|---|
| Related Catalog | |
| Target |
MEK:2-15 nM (EC50) |
| In Vitro | Refametinib R enantiomer is the R enantiomer of Refametinib . Refametinib R enantiomer is an inhibitor of MEK and is useful in treatment of cancer and other hyperproliferative diseases[1]. |
| Kinase Assay | A typical 25 μL assay contains 0.002 nmol MEK1, 0.02 nmol ERK2, 0.25 nmol MBP, 0.25 nmol unlabeled ATP, and 0.1 μCi [γ33P] ATP. The screening assay essentially comprised four additions. Five μL of diluted compound are dispensed to 96-well assay plates. Ten μL of 2.5× enzyme cocktail (MEKl and ERK2 only) are then added to each well followed by a pre- incubation for 30 minutes at ambient temperature. Ten μL of 2.5× substrate cocktail (labeled and unlabeled ATP plus MBP) are then added, followed by incubation for 60 minutes at ambient temperature. Finally, 100 μL of 10% trichloroacetic acid (TCA) are added and incubated for 30 minutes at room temperature to halt the reaction and precipitate radiolabeled protein products. Reaction products are harvested on glass fiber 96 well filter plates prewetted with water and 1% pyrophosphate. The filter plate is then washed 5 times with water. Water is displaced by absolute ethanol and the plate is allowed to air dry for 30 minutes at room temperature. A back seal is applied manually and 40 μL of scintillation cocktail are dispensed to each well. A top seal is applied and the plate is counted in the TopCount for two seconds per well. For certain experiments a truncated version of MEK that requires activation by Raf kinase are used[1]. |
| Cell Assay | Effects of compounds in the cell are determined by Western blotting for phosphorylated ERK. MDA-MB-231 breast cancer cells are plated in a 48 well plate at 20,000 cells per well and grown in a 37° humidified CO2 incubator. The following day, the growth media (DMEM+10% fetal bovine serum) is removed and replaced with starve media (DMEM+0.1% fetal bovine serum). Cells are incubated in the starve media for sixteen hours and then treated with a range of compound concentrations for thirty minutes. After incubation with compound, cells are stimulated with 100ng/mL EGF for five minutes. The cells are then lysed and analyzed by Western blot using a monoclonal antibody raised to phosphorylated ERK. The signal is amplified using a secondary antibody conjugated to a near-IR dye and detected on a Licor Odyssey scanner. The intensity of signal is quantitated and this data is used to generate dose response curves and EC50 calculations[1]. |
| References |
[1]. Andreas Maderna, et al. N-(arylamino)-sulfonamide inhibitors of mek. WO 2007014011 A2. |
| Density | 1.8±0.1 g/cm3 |
|---|---|
| Boiling Point | 566.9±60.0 °C at 760 mmHg |
| Molecular Formula | C19H20F3IN2O5S |
| Molecular Weight | 572.337 |
| Flash Point | 296.7±32.9 °C |
| Exact Mass | 572.008972 |
| PSA | 116.27000 |
| LogP | 4.78 |
| Vapour Pressure | 0.0±1.6 mmHg at 25°C |
| Index of Refraction | 1.660 |
| Cyclopropanesulfonamide, N-[3,4-difluoro-2-[(2-fluoro-4-iodophenyl)amino]-6-methoxyphenyl]-1-(2,3-dihydroxypropyl)- |
| Refametinib (R enantiomer) |
| N-{3,4-Difluoro-2-[(2-fluoro-4-iodophenyl)amino]-6-methoxyphenyl}-1-(2,3-dihydroxypropyl)cyclopropanesulfonamide |
| Refametinib |
| Refametinib R enantiomer |