Simeprevir
Modify Date: 2024-01-02 17:32:49
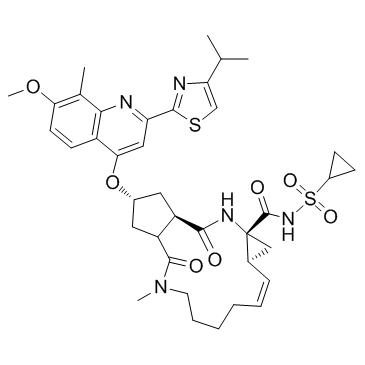
Simeprevir structure
|
Common Name | Simeprevir | ||
|---|---|---|---|---|
| CAS Number | 923604-59-5 | Molecular Weight | 749.939 | |
| Density | 1.4±0.1 g/cm3 | Boiling Point | N/A | |
| Molecular Formula | C38H47N5O7S2 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | N/A | |
Use of SimeprevirSimeprevir is a potent HCV NS3/4A protease inhibitor which suppresses HCV replication with EC50 of 8 nM. |
| Name | Simeprevir |
|---|---|
| Synonym | More Synonyms |
| Description | Simeprevir is a potent HCV NS3/4A protease inhibitor which suppresses HCV replication with EC50 of 8 nM. |
|---|---|
| Related Catalog | |
| Target |
EC50: 8 nM |
| In Vitro | In Huh7-Luc cells, antiviral activity of simeprevir (TMC435350) is dose dependent, and the EC50 and EC90 values determined for TMC435350 are 8 nM and 24 nM, respectively. Inhibition of TMC435350 on NS3/4A protease is time dependent, and the overall Kis are estimated to be 0.5 nM for genotype 1a and 0.4 nM for genotype 1b, respectively[1]. TMC435350 is a potent inhibitor of HCV NS3/4A protease (Ki=0.36 nM) and viral replication (replicon EC50=7.8 nM)[2]. |
| In Vivo | In rats, TMC435350 (40 mg/kg, p.o.) is extensively distributed to the liver and intestinal tract (tissue/plasma area under the concentration-time curve ratios of >35), and the absolute bioavailability is 44%[1]. |
| Kinase Assay | In vitro inhibition of NS3/4A activity is determined using a fluorescence resonance energy transfer cleavage assay with the RetS1 peptide substrate, derived from the genotype 1a NS4A-4B junction, and bacterially expressed full-length NS3 protease domain, supplemented with an NS4A peptide. Briefly, NS3/4A is preincubated in the presence of TMC435350 for 10 min, and then the RetS1 substrate is added and fluorescence is continuously measured for 20 min (excitation, 355 nm; emission, 500 nm). Cleavage of the substrate is expressed as a percentage of the cleavage seen with the vehicle control. |
| Cell Assay | Huh7-Luc cells are seeded at a density of 2,500 cells/well in a 384-well plate in Dulbecco's modified Eagle's medium plus 10% fetal calf serum and incubated with a range of concentrations of serially diluted simeprevir (TMC435350), in a final DMSO concentration of 0.5% in the absence of G418. After 72 h of incubation, Steady Lite reagent is added in a 1:1 ratio to the medium, and luciferase signal is measured using a ViewLux reader. |
| Animal Admin | Twenty-four male specific-pathogen-free Sprague-Dawley rats, weighing between 200 and 300 g at the time of dosing, are divided into eight groups of three rats each. Seven groups are dosed orally (p.o.) by gastric intubation of a vitamin E acetate-d-α-tocopheryl polyethylene glycol 1000 succinate-polyethylene glycol 400 solution of Simeprevir (TMC435350) at 2 mL/kg body weight to provide a dose of 40 mg/kg. One group is dosed intravenously (i.v.) by slow bolus injection in a tail vein of a 20% 2-hydroxypropyl-β-cyclodextrin formulation of TMC435350 (containing TMC435350, 100 mg/mL 2-hydroxypropyl-β-cyclodextrin, 0.1 N NaOH to pH 8.0±0.1, and mannitol-and pyrogen-free water) at 2 mL/kg body weight to provide a dose of 4 mg/kg. Water and food are available ad libitum during the study. |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Molecular Formula | C38H47N5O7S2 |
| Molecular Weight | 749.939 |
| Exact Mass | 749.291687 |
| PSA | 193.51000 |
| LogP | 4.99 |
| Index of Refraction | 1.653 |
| Storage condition | 2-8°C |
| (2R,11aS,12aR,14aR,Z)-N-(cyclopropylsulfonyl)-2-((2-(4-isopropylthiazol-2-yl)-7-methoxy-8-methylquinolin-4-yl)oxy)-5-methyl-4,14-dioxo-1,2,3,3a,4,5,6,7,8,9,11a,12,12a,13,14,14a-hexadecahydrocyclopenta[c]cyclopropa[g][1,6]diazacyclotetradecine-12a-carboxamide |
| Simeprevir |
| TMC435350 |
| TMC-435350 |
| Cyclopenta[c]cyclopropa[g][1,6]diazacyclotetradecine-12a(1H)-carboxamide, N-(cyclopropylsulfonyl)-2,3,3a,4,5,6,7,8,9,11a,12,13,14,14a-tetradecahydro-2-[[7-methoxy-8-methyl-2-[4-(1-methylethyl)-2-thiazolyl]-4-quinolinyl]oxy]-5-methyl-4,14-dioxo-, (2R,3aR,10Z,11aS,12aR,14aR)- |
| Olysio |
| (2R,3aR,10Z,11aS,12aR,14aR)-N-(Cyclopropylsulfonyl)-2-{[2-(4-isopropyl-1,3-thiazol-2-yl)-7-methoxy-8-methyl-4-quinolinyl]oxy}-5-methyl-4,14-dioxo-2,3,3a,4,5,6,7,8,9,11a,12,13,14,14a-tetradecahydrocyclopenta[c]cyclopropa[g][1,6]diazacyclotetradecine-12a(1H)-carboxamide |
| Olysio (TN) |
| (2R,3aR,10Z,11aS,12aR,14aR)-N-(cyclopropylsulfonyl)-2-({7-methoxy-8-methyl-2-[4-(1-methylethyl)thiazol-2-yl]quinolin-4-yl}oxy)-5-methyl-4,14-dioxo-2,3,3a,4,5,6,7,8,9,11a,12,13,14,14a-tetradecahydrocyclopenta[c]cyclopropa[g][1,6]diazacyclotetradecine-12a(1H)-carboxamide |
| TMC435 |