| Description |
S107 is a RyR-selective 1,4-benzothiazepine derivative that stabilizes RyR2 channels by enhancing the binding affinity of calstabin2 to mutant and/or PKA-phosphorylated channels.
|
| Related Catalog |
|
| In Vitro |
S107 is a small compound that enhances calstabin2 binding to RyR2 at low nanomolar concentrations and failed to interact with over 400 receptors, enzymes, and ion channels in screens using up to 10 μM of the compound. S107 has no effect on cardiac ion channels including the voltage-gated Na+, K+, and Ca2+ channels at concentrations up to 10 μM, and S107 had no effect on normal Ca2+ signaling in cells[1]. S107 is a promising candidate drug for treating catecholaminergic polymorphic ventricular tachycardia (CPVT). S107 exerts an antiarrhythmic effect on CPVT-hiPSC-CMs. Pre-incubation with 10 μM S107, which stabilizes the closed state of the ryanodine receptor 2, significantly decreases the percentage of CPVT-hiPSC-CMs presenting DADs to 25%[2]. S107 is thought to improve skeletal muscle function by stabilizing the RyR1-FKBP12 complex. S107 increases FKBP12 binding to RyR1 in SR vesicles in the presence of reduced glutathione and the NO-donor NOC12, with no effect in the presence of oxidized glutathione. S107 can reverse the harmful effects of redox active species on SR Ca2+ release in skeletal muscle by binding to RyR1 low affinity sites[3].
|
| In Vivo |
S107, which prevents a leak in the channel but does not block the channel or alter normal Ca2+ signaling, is able to inhibit both seizures and arrhythymias in the mutant mice[1].
|
| Animal Admin |
Mice: To test for protection against seizures using S107, osmotic pumps are implanted, and mice are pretreated with S107 5 mg/kg/h for 1 week prior to seizure susceptibility testing. Phase 4 seizures associated with death could be avoided through intubation and artificial breathing, indicating diaphragm failure during sustained seizures as a potential cause of death. Mice are directly observed and videorecorded for later review and latency classification during a 60-minute observation period[1].
|
| References |
[1]. Lehnart SE, et al. Leaky Ca2+ release channel/ryanodine receptor 2 causes seizures and sudden cardiac death in mice. J Clin Invest. 2008 Jun;118(6):2230-45. [2]. Sasaki K, et al. Patient-Specific Human Induced Pluripotent Stem Cell Model Assessed with Electrical Pacing Validates S107 as a Potential Therapeutic Agent for Catecholaminergic Polymorphic Ventricular Tachycardia. PLoS One. 2016 Oct 20;11(10):e0164795. [3]. Mei Y, et al. Stabilization of the skeletal muscle ryanodine receptor ion channel-FKBP12 complex by the 1,4-benzothiazepine derivative S107. PLoS One. 2013;8(1):e54208.
|
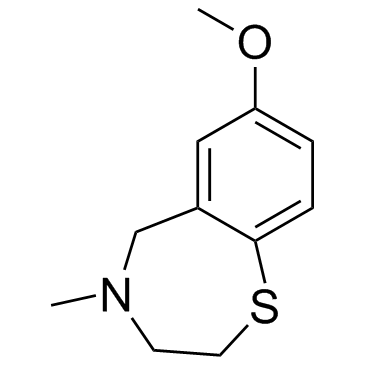

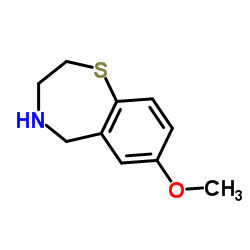


![3-[(4-METHOXYPHENYL)THIO]PROPANOIC ACID structure](https://image.chemsrc.com/caspic/069/13739-36-1.png)