CHEMICAL IDENTIFICATION
-
RTECS NUMBER :
-
DG2450000
-
CHEMICAL NAME :
-
Benzoic acid, p-amino-, ethyl ester
-
CAS REGISTRY NUMBER :
-
94-09-7
-
BEILSTEIN REFERENCE NO. :
-
0638434
-
LAST UPDATED :
-
199706
-
DATA ITEMS CITED :
-
12
-
MOLECULAR FORMULA :
-
C9-H11-N-O2
-
MOLECULAR WEIGHT :
-
165.21
-
WISWESSER LINE NOTATION :
-
ZR DVO2
HEALTH HAZARD DATA
ACUTE TOXICITY DATA
-
TYPE OF TEST :
-
Standard Draize test
-
ROUTE OF EXPOSURE :
-
Administration onto the skin
-
SPECIES OBSERVED :
-
Rodent - guinea pig
-
REFERENCE :
-
JSCCA5 Journal of the Society of Cosmetic Chemists. (Soc. of Cosmetic Chemists, 1995 Broadway, Suite 1701, New York, NY 10023) V.1- 1947- Volume(issue)/page/year: 28,357,1977 ** ACUTE TOXICITY DATA **
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Rectal
-
SPECIES OBSERVED :
-
Human - infant
-
DOSE/DURATION :
-
12 mg/kg
-
TOXIC EFFECTS :
-
Blood - methemoglobinemia-carboxyhemoglobin
-
REFERENCE :
-
NEJMAG New England Journal of Medicine. (Massachusetts Medical Soc., 10 Shattuck St., Boston, MA 02115) V.198- 1928- Volume(issue)/page/year: 263,454,1960
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
3042 mg/kg
-
TOXIC EFFECTS :
-
Behavioral - ataxia Lungs, Thorax, or Respiration - dyspnea Lungs, Thorax, or Respiration - cyanosis
-
REFERENCE :
-
TOVEFN Toksikologicheskii Vestnik. (18-20 Vadkovskii per. Moscow, 101479, Russia) History Unknown Volume(issue)/page/year: (2),34,1996
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
2500 mg/kg
-
TOXIC EFFECTS :
-
Behavioral - ataxia Lungs, Thorax, or Respiration - dyspnea Lungs, Thorax, or Respiration - cyanosis
-
REFERENCE :
-
TOVEFN Toksikologicheskii Vestnik. (18-20 Vadkovskii per. Moscow, 101479, Russia) History Unknown Volume(issue)/page/year: (2),34,1996
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
216 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
JMCMAR Journal of Medicinal Chemistry. (American Chemical Soc., Distribution Office Dept. 223, POB POB 57136, West End Stn., Washington, DC 20037) V.6- 1963- Volume(issue)/page/year: 17,900,1974
-
TYPE OF TEST :
-
LDLo - Lowest published lethal dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rabbit
-
DOSE/DURATION :
-
1150 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
NIIRDN Drugs in Japan (Ethical Drugs). (Yakugyo Jiho Co., Ltd., Tokyo, Japan) Volume(issue)/page/year: 6,33,1982
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Bird - wild bird species
-
DOSE/DURATION :
-
56 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
TXAPA9 Toxicology and Applied Pharmacology. (Academic Press, Inc., 1 E. First St., Duluth, MN 55802) V.1- 1959- Volume(issue)/page/year: 21,315,1972 *** REVIEWS *** TOXICOLOGY REVIEW PHREA7 Physiological Reviews. (American Physiological Soc., 9650 Rockville Pike, Bethesda, MD 20814) V.1- 1921- Volume(issue)/page/year: 12,190,1932 *** U.S. STANDARDS AND REGULATIONS *** EPA FIFRA 1988 PESTICIDE SUBJECT TO REGISTRATION OR RE-REGISTRATION FEREAC Federal Register. (U.S. Government Printing Office, Supt. of Documents, Washington, DC 20402) V.1- 1936- Volume(issue)/page/year: 54,7740,1989 *** NIOSH STANDARDS DEVELOPMENT AND SURVEILLANCE DATA *** NIOSH OCCUPATIONAL EXPOSURE SURVEY DATA : NOHS - National Occupational Hazard Survey (1974) NOHS Hazard Code - 81723 No. of Facilities: 293 (estimated) No. of Industries: 8 No. of Occupations: 7 No. of Employees: 2005 (estimated) NOES - National Occupational Exposure Survey (1983) NOES Hazard Code - 81723 No. of Facilities: 1499 (estimated) No. of Industries: 12 No. of Occupations: 17 No. of Employees: 167623 (estimated) No. of Female Employees: 146838 (estimated)
|
 CAS#:51934-41-9
CAS#:51934-41-9 CAS#:64-17-5
CAS#:64-17-5 CAS#:150-13-0
CAS#:150-13-0 CAS#:99-77-4
CAS#:99-77-4 CAS#:38556-93-3
CAS#:38556-93-3 CAS#:5798-75-4
CAS#:5798-75-4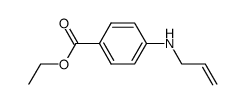 CAS#:73779-71-2
CAS#:73779-71-2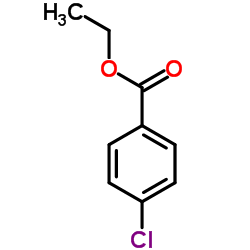 CAS#:7335-27-5
CAS#:7335-27-5 CAS#:14685-90-6
CAS#:14685-90-6![ethyl 4-[(4-nitrophenyl)methylideneamino]benzoate structure](https://image.chemsrc.com/caspic/411/108529-41-5.png) CAS#:108529-41-5
CAS#:108529-41-5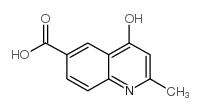 CAS#:103853-88-9
CAS#:103853-88-9 CAS#:110969-44-3
CAS#:110969-44-3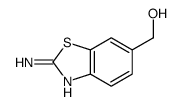 CAS#:106429-07-6
CAS#:106429-07-6 CAS#:10129-07-4
CAS#:10129-07-4 CAS#:555-06-6
CAS#:555-06-6 CAS#:582-80-9
CAS#:582-80-9![Benzoic acid,4-[(1,3-dioxobutyl)amino]-, ethyl ester structure](https://image.chemsrc.com/caspic/274/30764-23-9.png) CAS#:30764-23-9
CAS#:30764-23-9 CAS#:36151-44-7
CAS#:36151-44-7 CAS#:362527-61-5
CAS#:362527-61-5
