(R)-NADH-d1
Modify Date: 2024-08-05 09:37:39
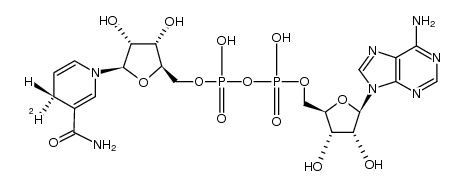
(R)-NADH-d1 structure
|
Common Name | (R)-NADH-d1 | ||
|---|---|---|---|---|
| CAS Number | 10012-96-1 | Molecular Weight | 666.44700 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C21H28DN7O14P2 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | N/A | |
Use of (R)-NADH-d1(R)-NADH-d1 is the deuterium labeled (R)-NADH. NADH is an orally active reduced coenzyme. NADH is a donor of ADP-ribose units in ADP-ribosylaton reactions and a precursor of cyclic ADP-ribose. NADH plays a role as a regenerative electron donor in cellular energy metabolism, including glycolysis, β-oxidation and the tricarboxylic acid (TCA) cycle[1][2]. |
| Name | [4r-(2h)]nadh |
|---|---|
| Synonym | More Synonyms |
| Description | (R)-NADH-d1 is the deuterium labeled (R)-NADH. NADH is an orally active reduced coenzyme. NADH is a donor of ADP-ribose units in ADP-ribosylaton reactions and a precursor of cyclic ADP-ribose. NADH plays a role as a regenerative electron donor in cellular energy metabolism, including glycolysis, β-oxidation and the tricarboxylic acid (TCA) cycle[1][2]. |
|---|---|
| Related Catalog | |
| In Vitro | Stable heavy isotopes of hydrogen, carbon, and other elements have been incorporated into drug molecules, largely as tracers for quantitation during the drug development process. Deuteration has gained attention because of its potential to affect the pharmacokinetic and metabolic profiles of drugs[1]. |
| Molecular Formula | C21H28DN7O14P2 |
|---|---|
| Molecular Weight | 666.44700 |
| Exact Mass | 666.13100 |
| PSA | 337.24000 |
| (4R)-[4-2H]-NADH |
| 4R-D-NADH |
| O5'-{2-[1-(3-carbamoyl-4H-pyridin-1-yl)-β-D-1-deoxy-ribofuranos-5-yloxy]-1,2-dihydroxy-phosphoryl}-adenosine |
| diphosphoric acid-1-adenosin-5'-yl ester-2-[(1R)-1-((4R)-3-carbamoyl-4-deuterio-4H-[1]pyridyl)-D-1,4-anhydro-ribitol-5-yl ester] |
| Diphosphorsaeure-1-adenosin-5'-ylester-2-[(1R)-1-((4R)-3-carbamoyl-4-deuterio-4H-[1]pyridyl)-D-1,4-anhydro-ribit-5-ylester] |
| [4R-2H]-NADH |