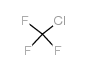
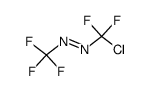
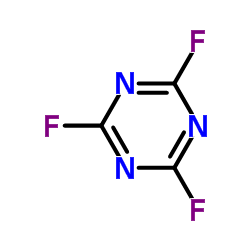
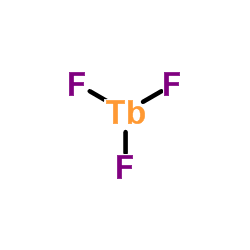
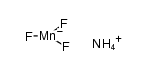7783-53-1
| 中文名 | 氟化锰(III) |
|---|---|
| 英文名 | manganese(iii) fluoride |
| 英文别名 |
EINECS 232-006-6
manganese (II)-oxalate manganese hematoporphyrinate manganese trifluoride manganous oxalate manganese hematoporphyrin-IX manganese(II) oxalatea MFCD00016223 Mangan(II)-oxalat |
| 密度 | 3.54 g/mL at 25ºC(lit.) |
|---|---|
| 沸点 | 19.5ºC at 760 mmHg |
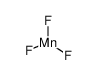
| 分子式 | F3Mn |
| 分子量 | 111.93300 |
| 精确质量 | 111.93300 |
| LogP | 1.26060 |
| 外观性状 | 紫色粉末或晶体 |
| 储存条件 | 保持贮藏器密封 放入紧密的贮藏器内,储存在阴凉,干燥的地方 |
| 稳定性 | 如果遵照规格使用和储存则不会分解 避免接触酸,水分/潮湿。红色晶体,600℃以上分解为二氟化锰和氟。与水发生水解反应,溶于酸,100mL HF在11.5℃下能溶解0.164g MnF3。在工业上和有机合成中用作氟化剂。 |
| 更多 | 1. 性状:未确定 2. 密度(g/mL 25ºC):3.54 3. 相对蒸汽密度(g/mL,空气=1):未确定 4. 熔点(ºC):未确定 5. 沸点(ºC,常压):未确定 6. 沸点(ºC,5.2kPa):未确定 7. 折射率(n20/D):未确定 8. 闪点(ºC,):未确定 9. 比旋光度(º):未确定 10. 自燃点或引燃温度(ºC):未确定 11. 蒸气压(kPa,25ºC):未确定 12. 饱和蒸气压(kPa,60ºC):未确定 13. 燃烧热(KJ/mol):未确定 14. 临界温度(ºC):未确定 15. 临界压力(KPa):未确定 16. 油水(辛醇/水)分配系数的对数值(25℃):未确定 17. 爆炸上限(%,V/V):未确定 18. 爆炸下限(%,V/V):未确定 19. 溶解性(mg/mL):未确定 |
Synonym: Manganese trifluoride; Manganic fluoride. SECTION 2 - COMPOSITION, INFORMATION ON INGREDIENTS
Risk Phrases: 36/37/38 SECTION 3 - HAZARDS IDENTIFICATION EMERGENCY OVERVIEW Irritating to eyes, respiratory system and skin.Moisture sensitive. Potential Health Effects Eye: Causes eye irritation. Skin: Causes skin irritation. Prolonged contact with the skin, especially if the skin is wet or moist, causes necrosis.
Ingestion: May cause irritation of the digestive tract. Inhalation: Causes respiratory tract irritation. Chronic: Chronic inhalation or ingestion may result in manganism characterized by neurological symptoms such as headache, apathy, and weakness of the legs, followed by psychosis and neurological symptoms similar to those of Parkinson's disease. May impair fertility. Other chronic effects from inhaling high amounts of manganese include an increased incidence of cough and bronchitis and susceptibility to infectious lung disease. SECTION 4 - FIRST AID MEASURES Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid immediately. Skin: Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Ingestion: Do not induce vomiting. If victim is conscious and alert, give 2-4 cupfuls of milk or water. Get medical aid immediately. Inhalation: Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. Get medical aid. Notes to Physician: SECTION 5 - FIRE FIGHTING MEASURES General Information: As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. Extinguishing Media: Use foam, dry chemical, or carbon dioxide. SECTION 6 - ACCIDENTAL RELEASE MEASURES General Information: Use proper personal protective equipment as indicated in Section 8. Spills/Leaks: Vacuum or sweep up material and place into a suitable disposal container. Avoid generating dusty conditions. Provide ventilation. Do not expose spill to water. Do not get water inside containers. SECTION 7 - HANDLING and STORAGE Handling: Use with adequate ventilation. Keep container tightly closed. Do not get on skin or in eyes. Avoid ingestion and inhalation. Storage: Store in a cool, dry place. Store in a tightly closed container. Store protected from moisture. SECTION 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION Engineering Controls: Use process enclosure, local exhaust ventilation, or other engineering controls to control airborne levels below recommended exposure limits. Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Exposure Limits CAS# 7783-53-1: United Kingdom, WEL - TWA: (listed as manganese, inorganic compounds): 0.5 mg/m3 TWA United Kingdom, WEL - STEL: (listed as manganese, inorganic compounds): 1.5 mg/m3 STEL United States OSHA: 5 mg/m3 Ceiling (as Mn) (listed under Mangan compounds, n.o.s.). Belgium - TWA: (listed as manganese compounds, n.o.s.): 0.2 mg/m3 (as Mn) Japan: (listed as manganese compounds, n.o.s.): 0.3 mg/m3 OEL (ex organic compounds, as Mn) Malaysia: (listed as manganese, inorganic compounds): 0.2 mg/m3 T (as Mn) Netherlands: (listed as manganese compounds, n.o.s.): 3 mg/m3 STE (as Mn) Netherlands: (listed as manganese compounds, n.o.s.): 1 mg/m3 MAC Mn) Spain: (listed as manganese, inorganic compounds): 0.2 mg/m3 VLA- (as Mn) Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166. Skin: Wear appropriate protective gloves to prevent skin exposure. Clothing: Wear appropriate protective clothing to prevent skin exposure. Respirators: Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced. SECTION 9 - PHYSICAL AND CHEMICAL PROPERTIES Physical State: Crystals Color: red-purple Odor: Not available. pH: Not available. Vapor Pressure: Not available. Viscosity: Not available. Boiling Point: Not available. Freezing/Melting Point: Not available. Autoignition Temperature: Not available. Flash Point: Not available. Explosion Limits, lower: Not available. Explosion Limits, upper: Not available. Decomposition Temperature: Solubility in water: Hydrolysis. Specific Gravity/Density: 3.54 Molecular Formula: F3Mn Molecular Weight: 111.93 SECTION 10 - STABILITY AND REACTIVITY Chemical Stability: Stable under normal temperatures and pressures. Conditions to Avoid: Moisture, excess heat. Incompatibilities with Other Materials: Strong bases, water, strong reducing agents, organic matter, combustible organics. Hazardous Decomposition Products: Fluoride fumes, oxides of manganese. Hazardous Polymerization: Has not been reported SECTION 11 - TOXICOLOGICAL INFORMATION RTECS#: CAS# 7783-53-1: OP0882600 LD50/LC50: CAS# 7783-53-1: Inhalation, mouse: LC50 = 320 mg/m3; Oral, mouse: LD50 = 86 mg/kg. Carcinogenicity: Manganese(III) fluoride - Not listed by ACGIH, IARC, or NTP. Other: See actual entry in RTECS for complete information. SECTION 12 - ECOLOGICAL INFORMATION SECTION 13 - DISPOSAL CONSIDERATIONS Dispose of in a manner consistent with federal, state, and local regulations. SECTION 14 - TRANSPORT INFORMATION IATA Not regulated as a hazardous material. IMO Not regulated as a hazardous material. RID/ADR Not regulated as a hazardous material. SECTION 15 - REGULATORY INFORMATION European/International Regulations European Labeling in Accordance with EC Directives Hazard Symbols: XI Risk Phrases: R 36/37/38 Irritating to eyes, respiratory system and skin. Safety Phrases: S 22 Do not breathe dust. S 24/25 Avoid contact with skin and eyes. S 36/37/39 Wear suitable protective clothing, gloves and eye/face protection. WGK (Water Danger/Protection) CAS# 7783-53-1: No information available. Canada CAS# 7783-53-1 is listed on Canada's NDSL List. CAS# 7783-53-1 is not listed on Canada's Ingredient Disclosure List. US FEDERAL TSCA CAS# 7783-53-1 is listed on the TSCA inventory. SECTION 16 - ADDITIONAL INFORMATION MSDS Creation Date: 1/27/1998 Revision #3 Date: 9/03/2004 The information above is believed to be accurate and represents the best information currently available to us. However, we make no warranty of merchantability or any other warranty, express or implied, with respect to such information, and we assume no liability resulting from its use. Users should make their own investigations to determine the suitability of the information for their particular purposes. In no way shall the company be liable for any claims, losses, or damages of any third party or for lost profits or any special, indirect, incidental, consequential or exemplary damages, howsoever arising, even if the company has been advised of the possibility of such damages. SECTION 16 - ADDITIONAL INFORMATION N/A |
|
生态学数据: 对水是稍微危害的,若无政府许可,勿将材料排入周围环境
|
| 符号 |


GHS03, GHS06 |
|---|---|
| 信号词 | Danger |
| 危害声明 | H272-H301-H312 + H332-H315-H319-H335 |
| 警示性声明 | Missing Phrase - N15.00950417-P210-P220-P261-P280-P370 + P378 |
| 个人防护装备 | dust mask type N95 (US);Eyeshields;Faceshields;full-face particle respirator type N100 (US);Gloves;respirator cartridge type N100 (US);type P1 (EN143) respirator filter;type P3 (EN 143) respirator cartridges |
| 危害码 (欧洲) | O,Xn |
| 风险声明 (欧洲) | 8-20/21/22-36/37/38 |
| 安全声明 (欧洲) | S17;S26;S36/S37/S39 |
| 危险品运输编码 | UN 3087 5.1/PG 2 |
| WGK德国 | 3 |
| RTECS号 | OP0882600 |
| 包装等级 | III |
| 危险类别 | 6.1 |
|
~% 
7783-53-1 |
| 文献:Zeitschrift fuer Anorganische und Allgemeine Chemie, , vol. 619, p. 1426 - 1430 |
|
~% 
7783-53-1 |
| 文献:Zeitschrift fuer Anorganische und Allgemeine Chemie, , vol. 376, p. 261 - 267 Ag: MVol.B4, 46.4.2, page 378 - 379 |
|
~% 
7783-53-1 |
| 文献:J. Prakt. Chem. (2), , vol. 35, p. 57 - 82 Mn: MVol.C1, 2.6.8.4.4, page 305 - 306 |
|
~% 
7783-53-1 |
| 文献:Russian Journal of Inorganic Chemistry, , vol. 42, # 11 p. 1646 - 1649 |
|
~% 
7783-53-1 |
| 文献:Russian Journal of Inorganic Chemistry, , vol. 42, # 2 p. 149 - 153 |
| 上游产品 5 | |
|---|---|
| 下游产品 7 | |