138483-63-3
| 中文名 | L-689502 |
|---|---|
| 英文名 | tert-butyl N-[(2S,3S,5R)-3-hydroxy-6-[[(1S,2R)-2-hydroxy-2,3-dihydro-1H-inden-1-yl]amino]-5-[[4-(2-morpholin-4-ylethoxy)phenyl]methyl]-6-oxo-1-phenylhexan-2-yl]carbamate |
| 英文别名 |
Des-3-pyridylmethyl Indinavir
(2S)-1-[(2S,4S)-4-benzyl-2-hydroxy-5-[[(1S,2R)-2-hydroxy-2,3-dihydro-1H-inden-1-yl]amino]-5-oxopentyl]-N-tert-butylpiperazine-2-carboxamide [14C]-N-Dealkyl Indinavir N-(2(R)-hydroxy-1(S)-indanyl)-2(R)-phenylmethyl-4(S)-hydroxy-5-(1-(2(S)-N-(tert-butylcarboxamido)piperazinyl))pentanamide N-(2(R)-hydroxy-1(S)-indanyl)-5(S)-[(tert-butyloxycarbonyl)amino]-4(S)-hydroxy-6-phenyl-2(R)-({4-[2-(4-morpholinyl)ethoxy]phenyl}methyl)hexanamide (1S,2R,2'R,4'S,2"S)-1-((2-benzyl-5-(((2-(t-butyl)amino)carbonyl)piperazin-1-yl)-4-hydroxypentanoyl)amino)indan-2-ol indinavir penultimate 2,3,5-Trideoxy-N-[(1S,2R)-2,3-dihydro-2-hydroxy-1H-inden-1-yl]-5-[(2S)-2-[[(1,1-dimethylethyl)amino]carbonyl]-1-piperazinyl]-2-(phenylmethyl)-D-erythro-pentonamide N-(2(R)-hydroxy-1(S)-indanyl)-5(S)-(1,1-dimethylethoxycarbonyl)amino-4(S)-hydroxy-6-phenyl-2(R)-(4-(2-(4-morpholinyl)ethoxy)phenyl)methyl hexanamide L-689502 |
| 描述 | L-689502 是一个有效的 HIV-1 蛋白酶 (HIV-l protease) 抑制剂,其 IC50 值为 1 nM。 |
|---|---|
| 相关类别 | |
| 靶点 |
IC50: 1 nM (HIV-l protease)[1] |
| 体外研究 | L694746和L-689502均以浓度依赖性方式抑制HIV-1蛋白酶活性。胃蛋白酶抑制剂的效力远低于显示IC50为2μM的任一化合物。 L694746在抑制HIV-1蛋白酶方面与L-689502一样有效,尽管其结构与L-689502不同[1]。 |
| 细胞实验 | 将G689502(在1mL的100%DMSO中的3.3pmole)加入已经生长48小时的培养物中。发酵期间的最终底物和DMSO浓度分别为65pM和2%。含有L-689502的发酵在收获前再进行72-96小时。用甲醇(0.5体积)和丙酮(0.5体积)将全发酵液进行间歇萃取。含有L-689502衍生物的上清液通过过滤从菌丝体中分离[1]。 |
| 参考文献 |
| 密度 | 1.24g/cm3 |
|---|---|
| 沸点 | 886.9ºC at 760 mmHg |
| 分子式 | C39H51N3O7 |
| 分子量 | 673.83800 |
| 闪点 | 490.2ºC |
| 精确质量 | 673.37300 |
| PSA | 136.57000 |
| LogP | 5.20040 |
| 蒸汽压 | 9.83E-34mmHg at 25°C |
| 折射率 | 1.615 |
| 储存条件 | 2-8℃ |
|
~99% 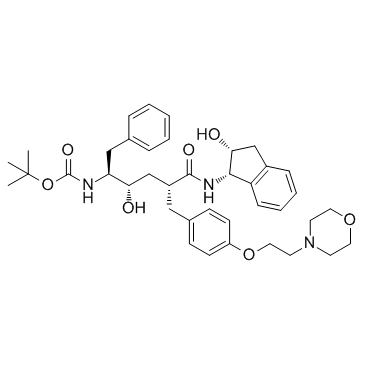
138483-63-3 |
| 文献:Thompson; Fitzgerald; Holloway; Emini; Darke; McKeever; Schleif; Quintero; Zugay; Tucker; Schwering; Homnick; Nunberg; Springer; Huff Journal of Medicinal Chemistry, 1992 , vol. 35, # 10 p. 1685 - 1701 |
|
~% 
138483-63-3 |
| 文献:Thompson; Fitzgerald; Holloway; Emini; Darke; McKeever; Schleif; Quintero; Zugay; Tucker; Schwering; Homnick; Nunberg; Springer; Huff Journal of Medicinal Chemistry, 1992 , vol. 35, # 10 p. 1685 - 1701 |
|
~% 
138483-63-3 |
| 文献:Thompson; Fitzgerald; Holloway; Emini; Darke; McKeever; Schleif; Quintero; Zugay; Tucker; Schwering; Homnick; Nunberg; Springer; Huff Journal of Medicinal Chemistry, 1992 , vol. 35, # 10 p. 1685 - 1701 |
|
~% 
138483-63-3 |
| 文献:Thompson; Fitzgerald; Holloway; Emini; Darke; McKeever; Schleif; Quintero; Zugay; Tucker; Schwering; Homnick; Nunberg; Springer; Huff Journal of Medicinal Chemistry, 1992 , vol. 35, # 10 p. 1685 - 1701 |
|
~% 
138483-63-3 |
| 文献:Thompson; Fitzgerald; Holloway; Emini; Darke; McKeever; Schleif; Quintero; Zugay; Tucker; Schwering; Homnick; Nunberg; Springer; Huff Journal of Medicinal Chemistry, 1992 , vol. 35, # 10 p. 1685 - 1701 |
|
~% 
138483-63-3 |
| 文献:Thompson; Fitzgerald; Holloway; Emini; Darke; McKeever; Schleif; Quintero; Zugay; Tucker; Schwering; Homnick; Nunberg; Springer; Huff Journal of Medicinal Chemistry, 1992 , vol. 35, # 10 p. 1685 - 1701 |
|
~% 
138483-63-3 |
| 文献:Askin, D.; Wallace, M. A.; Vacca, J. P.; Reamer, R. A.; Volante, R. P.; Shinkai, I. Journal of Organic Chemistry, 1992 , vol. 57, # 10 p. 2771 - 2773 |
|
~% 
138483-63-3 |
| 文献:Askin, D.; Wallace, M. A.; Vacca, J. P.; Reamer, R. A.; Volante, R. P.; Shinkai, I. Journal of Organic Chemistry, 1992 , vol. 57, # 10 p. 2771 - 2773 |
|
~% 
138483-63-3 |
| 文献:Askin, D.; Wallace, M. A.; Vacca, J. P.; Reamer, R. A.; Volante, R. P.; Shinkai, I. Journal of Organic Chemistry, 1992 , vol. 57, # 10 p. 2771 - 2773 |
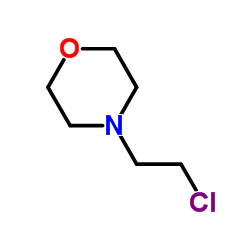
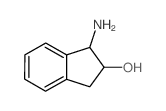
| 上游产品 3 | |
|---|---|
| 下游产品 0 | |