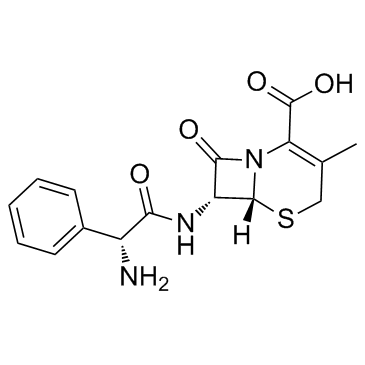
CHEMICAL IDENTIFICATION
-
RTECS NUMBER :
-
XI0350000
-
CHEMICAL NAME :
-
5-Thia-1-azabicyclo(4.2.0)oct-2-ene-2-carboxylic acid, 7-(2-amino-2-phenylacetamido)-3- methyl-8-oxo-, D-
-
CAS REGISTRY NUMBER :
-
15686-71-2
-
LAST UPDATED :
-
199612
-
DATA ITEMS CITED :
-
35
-
MOLECULAR FORMULA :
-
C16-H17-N3-O4-S
-
MOLECULAR WEIGHT :
-
347.42
HEALTH HAZARD DATA
ACUTE TOXICITY DATA
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Human
-
DOSE/DURATION :
-
14 mg/kg/D
-
TOXIC EFFECTS :
-
Gastrointestinal - hypermotility, diarrhea Gastrointestinal - nausea or vomiting
-
REFERENCE :
-
AACHAX Antimicrobial Agents and Chemotherapy (1961-70). (Ann Arbor, MI) 1961-70. For publisher information, see AMACCQ. Volume(issue)/page/year: -,361,1968
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
>20 gm/kg
-
TOXIC EFFECTS :
-
Behavioral - ataxia Skin and Appendages - hair Nutritional and Gross Metabolic - weight loss or decreased weight gain
-
REFERENCE :
-
NKRZAZ Chemotherapy (Tokyo). (Nippon Kagaku Ryoho Gakkai, 2-20-8 Kamiosaki, Shinagawa-Ku, Tokyo 141, Japan) V.1- 1953- Volume(issue)/page/year: 27(Suppl 7),765,1979
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
4 gm/kg
-
TOXIC EFFECTS :
-
Behavioral - excitement Behavioral - muscle weakness
-
REFERENCE :
-
KSRNAM Kiso to Rinsho. Clinical Report. (Yubunsha Co., Ltd., 1-5, Kanda Suda-Cho, Chiyoda-ku, KS Bldg., Tokyo 101, Japan) V.1- 1960- Volume(issue)/page/year: 3,390,1969
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
6100 mg/kg
-
TOXIC EFFECTS :
-
Behavioral - excitement Behavioral - muscle weakness Lungs, Thorax, or Respiration - respiratory depression
-
REFERENCE :
-
KSRNAM Kiso to Rinsho. Clinical Report. (Yubunsha Co., Ltd., 1-5, Kanda Suda-Cho, Chiyoda-ku, KS Bldg., Tokyo 101, Japan) V.1- 1960- Volume(issue)/page/year: 3,390,1969
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
1495 mg/kg
-
TOXIC EFFECTS :
-
Behavioral - changes in motor activity (specific assay) Skin and Appendages - hair
-
REFERENCE :
-
NKRZAZ Chemotherapy (Tokyo). (Nippon Kagaku Ryoho Gakkai, 2-20-8 Kamiosaki, Shinagawa-Ku, Tokyo 141, Japan) V.1- 1953- Volume(issue)/page/year: 27(Suppl 7),765,1979
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
400 mg/kg
-
TOXIC EFFECTS :
-
Sense Organs and Special Senses (Eye) - ptosis Kidney, Ureter, Bladder - urine volume increased Nutritional and Gross Metabolic - dehydration
-
REFERENCE :
-
AACHAX Antimicrobial Agents and Chemotherapy (1961-70). (Ann Arbor, MI) 1961-70. For publisher information, see AMACCQ. Volume(issue)/page/year: -,489,1968
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
1150 mg/kg
-
TOXIC EFFECTS :
-
Behavioral - altered sleep time (including change in righting reflex) Lungs, Thorax, or Respiration - other changes Kidney, Ureter, Bladder - other changes
-
REFERENCE :
-
OYYAA2 Oyo Yakuri. Pharmacometrics. (Oyo Yakuri Kenkyukai, CPO Box 180, Sendai 980-91, Japan) V.1- 1967- Volume(issue)/page/year: 3,227,1969
-
TYPE OF TEST :
-
LD - Lethal dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Mammal - dog
-
DOSE/DURATION :
-
>1 gm/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
KSRNAM Kiso to Rinsho. Clinical Report. (Yubunsha Co., Ltd., 1-5, Kanda Suda-Cho, Chiyoda-ku, KS Bldg., Tokyo 101, Japan) V.1- 1960- Volume(issue)/page/year: 3,513,1969
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Mammal - dog
-
DOSE/DURATION :
-
>1 gm/kg
-
TOXIC EFFECTS :
-
Behavioral - convulsions or effect on seizure threshold Behavioral - food intake (animal) Behavioral - ataxia
-
REFERENCE :
-
AACHAX Antimicrobial Agents and Chemotherapy (1961-70). (Ann Arbor, MI) 1961-70. For publisher information, see AMACCQ. Volume(issue)/page/year: -,489,1968
-
TYPE OF TEST :
-
LDLo - Lowest published lethal dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Primate - monkey
-
DOSE/DURATION :
-
>1 gm/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
AACHAX Antimicrobial Agents and Chemotherapy (1961-70). (Ann Arbor, MI) 1961-70. For publisher information, see AMACCQ. Volume(issue)/page/year: -,489,1968
-
TYPE OF TEST :
-
LDLo - Lowest published lethal dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Mammal - cat
-
DOSE/DURATION :
-
250 mg/kg
-
TOXIC EFFECTS :
-
Behavioral - somnolence (general depressed activity) Behavioral - food intake (animal) Gastrointestinal - nausea or vomiting
-
REFERENCE :
-
KSRNAM Kiso to Rinsho. Clinical Report. (Yubunsha Co., Ltd., 1-5, Kanda Suda-Cho, Chiyoda-ku, KS Bldg., Tokyo 101, Japan) V.1- 1960- Volume(issue)/page/year: 3,513,1969
-
TYPE OF TEST :
-
LDLo - Lowest published lethal dose
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Mammal - cat
-
DOSE/DURATION :
-
1 gm/kg
-
TOXIC EFFECTS :
-
Sense Organs and Special Senses (Eye) - lacrimation Behavioral - altered sleep time (including change in righting reflex) Nutritional and Gross Metabolic - weight loss or decreased weight gain
-
REFERENCE :
-
KSRNAM Kiso to Rinsho. Clinical Report. (Yubunsha Co., Ltd., 1-5, Kanda Suda-Cho, Chiyoda-ku, KS Bldg., Tokyo 101, Japan) V.1- 1960- Volume(issue)/page/year: 3,513,1969
-
TYPE OF TEST :
-
LDLo - Lowest published lethal dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rabbit
-
DOSE/DURATION :
-
2500 mg/kg
-
TOXIC EFFECTS :
-
Behavioral - food intake (animal) Gastrointestinal - hypermotility, diarrhea Nutritional and Gross Metabolic - weight loss or decreased weight gain
-
REFERENCE :
-
KSRNAM Kiso to Rinsho. Clinical Report. (Yubunsha Co., Ltd., 1-5, Kanda Suda-Cho, Chiyoda-ku, KS Bldg., Tokyo 101, Japan) V.1- 1960- Volume(issue)/page/year: 3,513,1969
-
TYPE OF TEST :
-
LDLo - Lowest published lethal dose
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - rabbit
-
DOSE/DURATION :
-
>4 gm/kg
-
TOXIC EFFECTS :
-
Gastrointestinal - hypermotility, diarrhea
-
REFERENCE :
-
AACHAX Antimicrobial Agents and Chemotherapy (1961-70). (Ann Arbor, MI) 1961-70. For publisher information, see AMACCQ. Volume(issue)/page/year: -,489,1968
-
TYPE OF TEST :
-
LDLo - Lowest published lethal dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - guinea pig
-
DOSE/DURATION :
-
5 gm/kg
-
TOXIC EFFECTS :
-
Gastrointestinal - hypermotility, diarrhea
-
REFERENCE :
-
KSRNAM Kiso to Rinsho. Clinical Report. (Yubunsha Co., Ltd., 1-5, Kanda Suda-Cho, Chiyoda-ku, KS Bldg., Tokyo 101, Japan) V.1- 1960- Volume(issue)/page/year: 3,513,1969
-
TYPE OF TEST :
-
LDLo - Lowest published lethal dose
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - guinea pig
-
DOSE/DURATION :
-
1 gm/kg
-
TOXIC EFFECTS :
-
Behavioral - altered sleep time (including change in righting reflex) Lungs, Thorax, or Respiration - cyanosis Nutritional and Gross Metabolic - body temperature decrease
-
REFERENCE :
-
KSRNAM Kiso to Rinsho. Clinical Report. (Yubunsha Co., Ltd., 1-5, Kanda Suda-Cho, Chiyoda-ku, KS Bldg., Tokyo 101, Japan) V.1- 1960- Volume(issue)/page/year: 3,513,1969 ** OTHER MULTIPLE DOSE TOXICITY DATA **
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
35 gm/kg/5W-I
-
TOXIC EFFECTS :
-
Kidney, Ureter, Bladder - other changes in urine composition Endocrine - changes in adrenal weight Blood - changes in serum composition (e.g. TP, bilirubin, cholesterol)
-
REFERENCE :
-
NKRZAZ Chemotherapy (Tokyo). (Nippon Kagaku Ryoho Gakkai, 2-20-8 Kamiosaki, Shinagawa-Ku, Tokyo 141, Japan) V.1- 1953- Volume(issue)/page/year: 34(Suppl 5),72,1986
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
140 gm/kg/35D-C
-
TOXIC EFFECTS :
-
Kidney, Ureter, Bladder - urine volume increased Endocrine - changes in spleen weight Blood - changes in serum composition (e.g. TP, bilirubin, cholesterol)
-
REFERENCE :
-
OYYAA2 Oyo Yakuri. Pharmacometrics. (Oyo Yakuri Kenkyukai, CPO Box 180, Sendai 980-91, Japan) V.1- 1967- Volume(issue)/page/year: 3,227,1969
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
182 gm/kg/26W-I
-
TOXIC EFFECTS :
-
Kidney, Ureter, Bladder - changes in bladder weight Endocrine - changes in adrenal weight Biochemical - Metabolism (Intermediary) - other proteins
-
REFERENCE :
-
NKRZAZ Chemotherapy (Tokyo). (Nippon Kagaku Ryoho Gakkai, 2-20-8 Kamiosaki, Shinagawa-Ku, Tokyo 141, Japan) V.1- 1953- Volume(issue)/page/year: 28(Suppl 3),116,1980
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
56 gm/kg/26D-I
-
TOXIC EFFECTS :
-
Kidney, Ureter, Bladder - urine volume increased Kidney, Ureter, Bladder - changes in bladder weight Blood - changes in serum composition (e.g. TP, bilirubin, cholesterol)
-
REFERENCE :
-
NKRZAZ Chemotherapy (Tokyo). (Nippon Kagaku Ryoho Gakkai, 2-20-8 Kamiosaki, Shinagawa-Ku, Tokyo 141, Japan) V.1- 1953- Volume(issue)/page/year: 28(Suppl 3),131,1980
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
182 gm/kg/13W-C
-
TOXIC EFFECTS :
-
Kidney, Ureter, Bladder - other changes in urine composition Blood - normocytic anemia Blood - changes in serum composition (e.g. TP, bilirubin, cholesterol)
-
REFERENCE :
-
NKRZAZ Chemotherapy (Tokyo). (Nippon Kagaku Ryoho Gakkai, 2-20-8 Kamiosaki, Shinagawa-Ku, Tokyo 141, Japan) V.1- 1953- Volume(issue)/page/year: 28(Suppl 3),103,1980
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
6 gm/kg/5D-I
-
TOXIC EFFECTS :
-
Kidney, Ureter, Bladder - other changes Kidney, Ureter, Bladder - changes in bladder weight Blood - changes in serum composition (e.g. TP, bilirubin, cholesterol)
-
REFERENCE :
-
KSRNAM Kiso to Rinsho. Clinical Report. (Yubunsha Co., Ltd., 1-5, Kanda Suda-Cho, Chiyoda-ku, KS Bldg., Tokyo 101, Japan) V.1- 1960- Volume(issue)/page/year: 3,468,1969
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
72800 mg/kg/13W-C
-
TOXIC EFFECTS :
-
Behavioral - fluid intake Liver - changes in liver weight Blood - normocytic anemia
-
REFERENCE :
-
NKRZAZ Chemotherapy (Tokyo). (Nippon Kagaku Ryoho Gakkai, 2-20-8 Kamiosaki, Shinagawa-Ku, Tokyo 141, Japan) V.1- 1953- Volume(issue)/page/year: 28(Suppl 3),103,1980
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Mammal - dog
-
DOSE/DURATION :
-
31 gm/kg/31D-I
-
TOXIC EFFECTS :
-
Gastrointestinal - changes in structure or function of salivary glands Gastrointestinal - hypermotility, diarrhea Gastrointestinal - nausea or vomiting
-
REFERENCE :
-
NKRZAZ Chemotherapy (Tokyo). (Nippon Kagaku Ryoho Gakkai, 2-20-8 Kamiosaki, Shinagawa-Ku, Tokyo 141, Japan) V.1- 1953- Volume(issue)/page/year: 27(Suppl 7),812,1979
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rabbit
-
DOSE/DURATION :
-
2100 mg/kg/7D-I
-
TOXIC EFFECTS :
-
Kidney, Ureter, Bladder - changes in tubules (including acute renal failure, acute tubular necrosis) Kidney, Ureter, Bladder - other changes in urine composition Nutritional and Gross Metabolic - changes in chlorine
-
REFERENCE :
-
KSRNAM Kiso to Rinsho. Clinical Report. (Yubunsha Co., Ltd., 1-5, Kanda Suda-Cho, Chiyoda-ku, KS Bldg., Tokyo 101, Japan) V.1- 1960- Volume(issue)/page/year: 29,3459,1995
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Rodent - rabbit
-
DOSE/DURATION :
-
10 gm/kg/5D-I
-
TOXIC EFFECTS :
-
Kidney, Ureter, Bladder - changes in tubules (including acute renal failure, acute tubular necrosis) Kidney, Ureter, Bladder - urine volume increased Kidney, Ureter, Bladder - changes in bladder weight
-
REFERENCE :
-
NKRZAZ Chemotherapy (Tokyo). (Nippon Kagaku Ryoho Gakkai, 2-20-8 Kamiosaki, Shinagawa-Ku, Tokyo 141, Japan) V.1- 1953- Volume(issue)/page/year: 28(Suppl 3),131,1980 ** REPRODUCTIVE DATA **
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
22 gm/kg
-
SEX/DURATION :
-
female 7-17 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - other measures of fertility Reproductive - Effects on Embryo or Fetus - fetotoxicity (except death, e.g., stunted fetus)
-
REFERENCE :
-
NKRZAZ Chemotherapy (Tokyo). (Nippon Kagaku Ryoho Gakkai, 2-20-8 Kamiosaki, Shinagawa-Ku, Tokyo 141, Japan) V.1- 1953- Volume(issue)/page/year: 27(Suppl 7),846,1979
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
52 gm/kg
-
SEX/DURATION :
-
female 17-22 day(s) after conception lactating female 20 day(s) post-birth
-
TOXIC EFFECTS :
-
Reproductive - Effects on Newborn - physical
-
REFERENCE :
-
NKRZAZ Chemotherapy (Tokyo). (Nippon Kagaku Ryoho Gakkai, 2-20-8 Kamiosaki, Shinagawa-Ku, Tokyo 141, Japan) V.1- 1953- Volume(issue)/page/year: 27(Suppl 7),865,1979
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
4 gm/kg
-
SEX/DURATION :
-
female 9-14 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Embryo or Fetus - extra-embryonic structures (e.g., placenta, umbilical cord) Reproductive - Effects on Embryo or Fetus - fetotoxicity (except death, e.g., stunted fetus) Reproductive - Effects on Newborn - physical
-
REFERENCE :
-
OYYAA2 Oyo Yakuri. Pharmacometrics. (Oyo Yakuri Kenkyukai, CPO Box 180, Sendai 980-91, Japan) V.1- 1967- Volume(issue)/page/year: 3,249,1969
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
32 gm/kg
-
SEX/DURATION :
-
female 9-14 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Maternal Effects - ovaries, fallopian tubes Reproductive - Specific Developmental Abnormalities - musculoskeletal system
-
REFERENCE :
-
OYYAA2 Oyo Yakuri. Pharmacometrics. (Oyo Yakuri Kenkyukai, CPO Box 180, Sendai 980-91, Japan) V.1- 1967- Volume(issue)/page/year: 3,249,1969
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
10 gm/kg
-
SEX/DURATION :
-
female 6-15 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Embryo or Fetus - fetotoxicity (except death, e.g., stunted fetus) Reproductive - Specific Developmental Abnormalities - musculoskeletal system
-
REFERENCE :
-
NKRZAZ Chemotherapy (Tokyo). (Nippon Kagaku Ryoho Gakkai, 2-20-8 Kamiosaki, Shinagawa-Ku, Tokyo 141, Japan) V.1- 1953- Volume(issue)/page/year: 27(Suppl 7),846,1979
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
600 mg/kg
-
SEX/DURATION :
-
female 7-12 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Maternal Effects - uterus, cervix, vagina Reproductive - Specific Developmental Abnormalities - musculoskeletal system
-
REFERENCE :
-
OYYAA2 Oyo Yakuri. Pharmacometrics. (Oyo Yakuri Kenkyukai, CPO Box 180, Sendai 980-91, Japan) V.1- 1967- Volume(issue)/page/year: 3,249,1969
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
4800 mg/kg
-
SEX/DURATION :
-
female 7-12 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Embryo or Fetus - extra-embryonic structures (e.g., placenta, umbilical cord) Reproductive - Effects on Newborn - physical
-
REFERENCE :
-
OYYAA2 Oyo Yakuri. Pharmacometrics. (Oyo Yakuri Kenkyukai, CPO Box 180, Sendai 980-91, Japan) V.1- 1967- Volume(issue)/page/year: 3,249,1969 *** NIOSH STANDARDS DEVELOPMENT AND SURVEILLANCE DATA *** NIOSH OCCUPATIONAL EXPOSURE SURVEY DATA : NOHS - National Occupational Hazard Survey (1974) NOHS Hazard Code - 83992 No. of Facilities: 58 (estimated) No. of Industries: 2 No. of Occupations: 3 No. of Employees: 902 (estimated) NOES - National Occupational Exposure Survey (1983) NOES Hazard Code - 83992 No. of Facilities: 171 (estimated) No. of Industries: 1 No. of Occupations: 3 No. of Employees: 2977 (estimated) No. of Female Employees: 1729 (estimated)
|