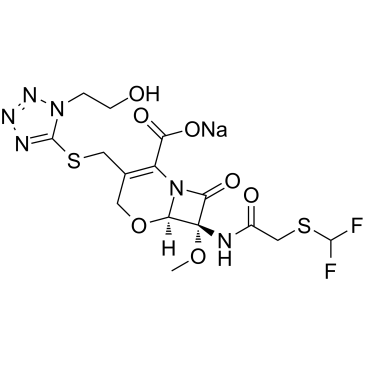
CHEMICAL IDENTIFICATION
-
RTECS NUMBER :
-
RN6900000
-
CHEMICAL NAME :
-
5-Oxa-1-azabicyclo(4.2.0)oct-2-ene-2-carboxylic acid, 7-(2-((difluoromethyl)thio)acetamido)- 3-(((1-(2-hydroxyethyl)-1H-tetrazol-5-yl)thio)methyl) -7-methoxy-8-oxo-, sodium salt, (6R,7R)-
-
CAS REGISTRY NUMBER :
-
92823-03-5
-
LAST UPDATED :
-
199706
-
DATA ITEMS CITED :
-
21
-
MOLECULAR FORMULA :
-
C15-H18-F2-N6-O7-S2.Na
-
MOLECULAR WEIGHT :
-
519.50
HEALTH HAZARD DATA
ACUTE TOXICITY DATA
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
>10 gm/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
IYKEDH Iyakuhin Kenkyu. Study of Medical Supplies. (Nippon Koteisho Kyokai, 12-15, 2-chome, Shibuya, Shibuya-ku, Tokyo 150, Japan) V.1- 1970- Volume(issue)/page/year: 19,735,1988
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
>10 gm/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
NIIRDN Drugs in Japan (Ethical Drugs). (Yakugyo Jiho Co., Ltd., Tokyo, Japan) Volume(issue)/page/year: -,1055,1990
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
6417 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
NIIRDN Drugs in Japan (Ethical Drugs). (Yakugyo Jiho Co., Ltd., Tokyo, Japan) Volume(issue)/page/year: -,1055,1990
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
>15 gm/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
IYKEDH Iyakuhin Kenkyu. Study of Medical Supplies. (Nippon Koteisho Kyokai, 12-15, 2-chome, Shibuya, Shibuya-ku, Tokyo 150, Japan) V.1- 1970- Volume(issue)/page/year: 19,735,1988
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
13500 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
IYKEDH Iyakuhin Kenkyu. Study of Medical Supplies. (Nippon Koteisho Kyokai, 12-15, 2-chome, Shibuya, Shibuya-ku, Tokyo 150, Japan) V.1- 1970- Volume(issue)/page/year: 19,735,1988
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
8043 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
IYKEDH Iyakuhin Kenkyu. Study of Medical Supplies. (Nippon Koteisho Kyokai, 12-15, 2-chome, Shibuya, Shibuya-ku, Tokyo 150, Japan) V.1- 1970- Volume(issue)/page/year: 19,735,1988
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Mammal - dog
-
DOSE/DURATION :
-
>5 gm/kg
-
TOXIC EFFECTS :
-
Gastrointestinal - changes in structure or function of salivary glands Gastrointestinal - hypermotility, diarrhea Gastrointestinal - nausea or vomiting
-
REFERENCE :
-
NKRZAZ Chemotherapy (Tokyo). (Nippon Kagaku Ryoho Gakkai, 2-20-8 Kamiosaki, Shinagawa-Ku, Tokyo 141, Japan) V.1- 1953- Volume(issue)/page/year: 35(Suppl 1),226,1987 ** OTHER MULTIPLE DOSE TOXICITY DATA **
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
90 gm/kg/30D-C
-
TOXIC EFFECTS :
-
Kidney, Ureter, Bladder - changes in bladder weight Blood - changes in leukocyte (WBC) count Nutritional and Gross Metabolic - changes in calcium
-
REFERENCE :
-
NKRZAZ Chemotherapy (Tokyo). (Nippon Kagaku Ryoho Gakkai, 2-20-8 Kamiosaki, Shinagawa-Ku, Tokyo 141, Japan) V.1- 1953- Volume(issue)/page/year: 32(Suppl 3),199,1984
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
273 gm/kg/26W-C
-
TOXIC EFFECTS :
-
Blood - changes in serum composition (e.g. TP, bilirubin, cholesterol) Nutritional and Gross Metabolic - weight loss or decreased weight gain Related to Chronic Data - changes in testicular weight
-
REFERENCE :
-
NKRZAZ Chemotherapy (Tokyo). (Nippon Kagaku Ryoho Gakkai, 2-20-8 Kamiosaki, Shinagawa-Ku, Tokyo 141, Japan) V.1- 1953- Volume(issue)/page/year: 32(Suppl 3),199,1984
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
146 gm/kg/26W-I
-
TOXIC EFFECTS :
-
Gastrointestinal - other changes Kidney, Ureter, Bladder - changes in bladder weight Endocrine - changes in adrenal weight
-
REFERENCE :
-
NKRZAZ Chemotherapy (Tokyo). (Nippon Kagaku Ryoho Gakkai, 2-20-8 Kamiosaki, Shinagawa-Ku, Tokyo 141, Japan) V.1- 1953- Volume(issue)/page/year: 35(Suppl 1),270,1987
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
90 mg/kg/30D-I
-
TOXIC EFFECTS :
-
Gastrointestinal - other changes Endocrine - changes in adrenal weight Biochemical - Enzyme inhibition, induction, or change in blood or tissue levels - transaminases
-
REFERENCE :
-
NKRZAZ Chemotherapy (Tokyo). (Nippon Kagaku Ryoho Gakkai, 2-20-8 Kamiosaki, Shinagawa-Ku, Tokyo 141, Japan) V.1- 1953- Volume(issue)/page/year: 35(Suppl 1),207,1987
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Mammal - dog
-
DOSE/DURATION :
-
15 gm/kg/30D-I
-
TOXIC EFFECTS :
-
Liver - changes in liver weight Kidney, Ureter, Bladder - changes in bladder weight Biochemical - Metabolism (Intermediary) - lipids including transport
-
REFERENCE :
-
NKRZAZ Chemotherapy (Tokyo). (Nippon Kagaku Ryoho Gakkai, 2-20-8 Kamiosaki, Shinagawa-Ku, Tokyo 141, Japan) V.1- 1953- Volume(issue)/page/year: 35(Suppl 1),226,1987
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Mammal - dog
-
DOSE/DURATION :
-
36 gm/kg/13W-I
-
TOXIC EFFECTS :
-
Blood - change in clotting factors Blood - changes in serum composition (e.g. TP, bilirubin, cholesterol) Biochemical - Enzyme inhibition, induction, or change in blood or tissue levels - phosphatases
-
REFERENCE :
-
NKRZAZ Chemotherapy (Tokyo). (Nippon Kagaku Ryoho Gakkai, 2-20-8 Kamiosaki, Shinagawa-Ku, Tokyo 141, Japan) V.1- 1953- Volume(issue)/page/year: 35(Suppl 1),251,1987 ** REPRODUCTIVE DATA **
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intravenous
-
DOSE :
-
41600 mg/kg
-
SEX/DURATION :
-
female 17-22 day(s) after conception lactating female 20 day(s) post-birth
-
TOXIC EFFECTS :
-
Reproductive - Fertility - litter size (e.g. # fetuses per litter; measured before birth) Reproductive - Effects on Newborn - live birth index (measured after birth) Reproductive - Effects on Newborn - growth statistics (e.g.%, reduced weight gain)
-
REFERENCE :
-
NKRZAZ Chemotherapy (Tokyo). (Nippon Kagaku Ryoho Gakkai, 2-20-8 Kamiosaki, Shinagawa-Ku, Tokyo 141, Japan) V.1- 1953- Volume(issue)/page/year: 35(Suppl 1),404,1987
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intravenous
-
DOSE :
-
10400 mg/kg
-
SEX/DURATION :
-
female 17-22 day(s) after conception lactating female 20 day(s) post-birth
-
TOXIC EFFECTS :
-
Reproductive - Maternal Effects - ovaries, fallopian tubes
-
REFERENCE :
-
NKRZAZ Chemotherapy (Tokyo). (Nippon Kagaku Ryoho Gakkai, 2-20-8 Kamiosaki, Shinagawa-Ku, Tokyo 141, Japan) V.1- 1953- Volume(issue)/page/year: 35(Suppl 1),404,1987
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intravenous
-
DOSE :
-
21 gm/kg
-
SEX/DURATION :
-
female 17-22 day(s) after conception lactating female 20 day(s) post-birth
-
TOXIC EFFECTS :
-
Reproductive - Maternal Effects - uterus, cervix, vagina
-
REFERENCE :
-
NKRZAZ Chemotherapy (Tokyo). (Nippon Kagaku Ryoho Gakkai, 2-20-8 Kamiosaki, Shinagawa-Ku, Tokyo 141, Japan) V.1- 1953- Volume(issue)/page/year: 35(Suppl 1),404,1987
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intravenous
-
DOSE :
-
8800 mg/kg
-
SEX/DURATION :
-
female 7-17 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Embryo or Fetus - extra-embryonic structures (e.g., placenta, umbilical cord) Reproductive - Effects on Embryo or Fetus - fetotoxicity (except death, e.g., stunted fetus) Reproductive - Effects on Newborn - viability index (e.g., # alive at day 4 per # born alive)
-
REFERENCE :
-
NKRZAZ Chemotherapy (Tokyo). (Nippon Kagaku Ryoho Gakkai, 2-20-8 Kamiosaki, Shinagawa-Ku, Tokyo 141, Japan) V.1- 1953- Volume(issue)/page/year: 35(Suppl 1),370,1987
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
DOSE :
-
2 gm/kg
-
SEX/DURATION :
-
female 6-15 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Embryo or Fetus - fetotoxicity (except death, e.g., stunted fetus) Reproductive - Specific Developmental Abnormalities - musculoskeletal system
-
REFERENCE :
-
OYYAA2 Oyo Yakuri. Pharmacometrics. (Oyo Yakuri Kenkyukai, CPO Box 180, Sendai 980-91, Japan) V.1- 1967- Volume(issue)/page/year: 34,335,1987
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
DOSE :
-
8 gm/kg
-
SEX/DURATION :
-
female 6-15 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - litter size (e.g. # fetuses per litter; measured before birth) Reproductive - Effects on Embryo or Fetus - other effects to embryo
-
REFERENCE :
-
OYYAA2 Oyo Yakuri. Pharmacometrics. (Oyo Yakuri Kenkyukai, CPO Box 180, Sendai 980-91, Japan) V.1- 1967- Volume(issue)/page/year: 34,335,1987
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intravenous
-
DOSE :
-
81 mg/kg
-
SEX/DURATION :
-
female 6-18 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Embryo or Fetus - fetal death
-
REFERENCE :
-
OYYAA2 Oyo Yakuri. Pharmacometrics. (Oyo Yakuri Kenkyukai, CPO Box 180, Sendai 980-91, Japan) V.1- 1967- Volume(issue)/page/year: 34,475,1987
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intravenous
-
DOSE :
-
19500 ug/kg
-
SEX/DURATION :
-
female 6-18 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Embryo or Fetus - fetotoxicity (except death, e.g., stunted fetus) Reproductive - Specific Developmental Abnormalities - musculoskeletal system
-
REFERENCE :
-
OYYAA2 Oyo Yakuri. Pharmacometrics. (Oyo Yakuri Kenkyukai, CPO Box 180, Sendai 980-91, Japan) V.1- 1967- Volume(issue)/page/year: 34,475,1987
|