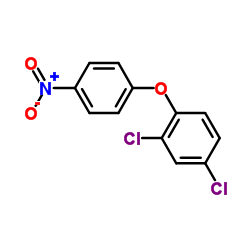
生态学数据:
对水是极其危害的,即使是少量产品渗入地下也会对饮用水造成危害,若无政府许可勿将产品排入周围环境。对水中有机物有危害
CHEMICAL IDENTIFICATION
-
RTECS NUMBER :
-
KN8400000
-
CHEMICAL NAME :
-
Ether, 2,4-dichlorophenyl p-nitrophenyl
-
CAS REGISTRY NUMBER :
-
1836-75-5
-
BEILSTEIN REFERENCE NO. :
-
1887356
-
LAST UPDATED :
-
199710
-
DATA ITEMS CITED :
-
64
-
MOLECULAR FORMULA :
-
C12-H7-Cl2-N-O3
-
MOLECULAR WEIGHT :
-
284.10
-
WISWESSER LINE NOTATION :
-
WNR DOR BG DG
HEALTH HAZARD DATA
ACUTE TOXICITY DATA
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
740 mg/kg
-
TOXIC EFFECTS :
-
Behavioral - excitement Lungs, Thorax, or Respiration - respiratory stimulation Nutritional and Gross Metabolic - body temperature increase
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Administration onto the skin
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
5 gm/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Unreported
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
3 gm/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
450 mg/kg
-
TOXIC EFFECTS :
-
Behavioral - excitement Lungs, Thorax, or Respiration - respiratory stimulation Nutritional and Gross Metabolic - body temperature increase
-
TYPE OF TEST :
-
LDLo - Lowest published lethal dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Mammal - cat
-
DOSE/DURATION :
-
300 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LCLo - Lowest published lethal concentration
-
ROUTE OF EXPOSURE :
-
Inhalation
-
SPECIES OBSERVED :
-
Mammal - cat
-
DOSE/DURATION :
-
620 mg/m3/4H
-
TOXIC EFFECTS :
-
Behavioral - excitement Lungs, Thorax, or Respiration - dyspnea Gastrointestinal - changes in structure or function of salivary glands
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rabbit
-
DOSE/DURATION :
-
1620 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LDLo - Lowest published lethal dose
-
ROUTE OF EXPOSURE :
-
Administration onto the skin
-
SPECIES OBSERVED :
-
Rodent - rabbit
-
DOSE/DURATION :
-
3270 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
13650 mg/kg/13W-C
-
TOXIC EFFECTS :
-
Liver - changes in liver weight Kidney, Ureter, Bladder - changes in bladder weight Related to Chronic Data - changes in testicular weight
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
40740 mg/kg/13W-C
-
TOXIC EFFECTS :
-
Liver - changes in liver weight Kidney, Ureter, Bladder - changes in bladder weight
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
40740 mg/kg/97W-C
-
TOXIC EFFECTS :
-
Liver - changes in liver weight Kidney, Ureter, Bladder - changes in bladder weight Related to Chronic Data - death
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Mammal - dog
-
DOSE/DURATION :
-
1400 mg/kg/4W-C
-
TOXIC EFFECTS :
-
Liver - changes in liver weight
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Mammal - dog
-
DOSE/DURATION :
-
36400 mg/kg/2Y-C
-
TOXIC EFFECTS :
-
Liver - changes in liver weight
-
TYPE OF TEST :
-
TCLo - Lowest published toxic concentration
-
ROUTE OF EXPOSURE :
-
Inhalation
-
SPECIES OBSERVED :
-
Mammal - cat
-
DOSE/DURATION :
-
26 mg/m3/4H/7W-I
-
TOXIC EFFECTS :
-
Blood - pigmented or nucleated red blood cells Biochemical - Enzyme inhibition, induction, or change in blood or tissue levels - catalases Related to Chronic Data - death
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
42 gm/kg/94W-C
-
TOXIC EFFECTS :
-
Tumorigenic - Carcinogenic by RTECS criteria Vascular - tumors Liver - tumors
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
24 gm/kg/12W-C
-
TOXIC EFFECTS :
-
Tumorigenic - Carcinogenic by RTECS criteria Liver - tumors Lungs, Thorax, or Respiration - tumors
-
TYPE OF TEST :
-
TD - Toxic dose (other than lowest)
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
200 gm/kg/78W-C
-
TOXIC EFFECTS :
-
Tumorigenic - Carcinogenic by RTECS criteria Vascular - tumors Liver - tumors
-
TYPE OF TEST :
-
TD - Toxic dose (other than lowest)
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
308 gm/kg/78W-C
-
TOXIC EFFECTS :
-
Tumorigenic - Carcinogenic by RTECS criteria Vascular - tumors Liver - tumors
-
TYPE OF TEST :
-
TD - Toxic dose (other than lowest)
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
114 gm/kg/58W-C
-
TOXIC EFFECTS :
-
Tumorigenic - Carcinogenic by RTECS criteria Vascular - tumors Liver - tumors
-
TYPE OF TEST :
-
TD - Toxic dose (other than lowest)
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
47 gm/kg/12W-C
-
TOXIC EFFECTS :
-
Tumorigenic - Carcinogenic by RTECS criteria Liver - tumors Lungs, Thorax, or Respiration - tumors
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
62500 ug/kg
-
SEX/DURATION :
-
female 6-15 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - body wall Reproductive - Specific Developmental Abnormalities - respiratory system Reproductive - Specific Developmental Abnormalities - urogenital system
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
150 mg/kg
-
SEX/DURATION :
-
female 7-12 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Embryo or Fetus - cytological changes (including somatic cell genetic material)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
37530 ug/kg
-
SEX/DURATION :
-
female 6-18 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Newborn - physical
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
62500 ug/kg
-
SEX/DURATION :
-
female 6-15 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Embryo or Fetus - fetotoxicity (except death, e.g., stunted fetus)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
100 mg/kg
-
SEX/DURATION :
-
female 11 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Newborn - growth statistics (e.g.%, reduced weight gain)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
80 mg/kg
-
SEX/DURATION :
-
female 10-13 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - cardiovascular (circulatory) system Reproductive - Effects on Newborn - stillbirth Reproductive - Effects on Newborn - viability index (e.g., # alive at day 4 per # born alive)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Administration onto the skin
-
DOSE :
-
12 mg/kg
-
SEX/DURATION :
-
female 6-15 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - eye/ear Reproductive - Specific Developmental Abnormalities - respiratory system
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Administration onto the skin
-
DOSE :
-
120 mg/kg
-
SEX/DURATION :
-
female 6-15 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - body wall Reproductive - Specific Developmental Abnormalities - endocrine system Reproductive - Specific Developmental Abnormalities - urogenital system
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Administration onto the skin
-
DOSE :
-
120 mg/kg
-
SEX/DURATION :
-
female 6-15 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Newborn - viability index (e.g., # alive at day 4 per # born alive) Reproductive - Effects on Newborn - growth statistics (e.g.%, reduced weight gain)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Unreported
-
DOSE :
-
13900 ug/kg
-
SEX/DURATION :
-
female 7-16 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - body wall
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Unreported
-
DOSE :
-
125 mg/kg
-
SEX/DURATION :
-
female 7-16 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - eye/ear
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Unreported
-
DOSE :
-
220 mg/kg
-
SEX/DURATION :
-
female 8-18 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - respiratory system
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Unreported
-
DOSE :
-
352 mg/kg
-
SEX/DURATION :
-
female 8-18 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Newborn - viability index (e.g., # alive at day 4 per # born alive)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
660 mg/kg
-
SEX/DURATION :
-
female 5-15 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - respiratory system Reproductive - Effects on Newborn - weaning or lactation index (e.g., # alive at weaning per # alive at day 4)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
1100 mg/kg
-
SEX/DURATION :
-
female 7-17 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Newborn - viability index (e.g., # alive at day 4 per # born alive) Reproductive - Effects on Newborn - physical Reproductive - Effects on Newborn - delayed effects
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
660 mg/kg
-
SEX/DURATION :
-
female 5-15 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Embryo or Fetus - fetotoxicity (except death, e.g., stunted fetus) Reproductive - Effects on Newborn - physical
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
68750 ug/kg
-
SEX/DURATION :
-
female 7-17 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - other developmental abnormalities Reproductive - Effects on Newborn - physical
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
550 mg/kg
-
SEX/DURATION :
-
female 7-17 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Newborn - viability index (e.g., # alive at day 4 per # born alive)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
2520 mg/kg
-
SEX/DURATION :
-
female 1-21 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - body wall Reproductive - Specific Developmental Abnormalities - respiratory system Reproductive - Specific Developmental Abnormalities - hepatobiliary system
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Administration onto the skin
-
DOSE :
-
175 mg/kg
-
SEX/DURATION :
-
female 8 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Newborn - other neonatal measures or effects
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Administration onto the skin
-
DOSE :
-
160 mg/kg
-
SEX/DURATION :
-
female 8 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Newborn - viability index (e.g., # alive at day 4 per # born alive)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Administration onto the skin
-
DOSE :
-
286 mg/kg
-
SEX/DURATION :
-
female 8 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - endocrine system
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Administration onto the skin
-
DOSE :
-
1100 mg/kg
-
SEX/DURATION :
-
female 7-17 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Newborn - viability index (e.g., # alive at day 4 per # born alive)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Unreported
-
DOSE :
-
550 mg/kg
-
SEX/DURATION :
-
female 7-17 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - eye/ear
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Unreported
-
DOSE :
-
1100 mg/kg
-
SEX/DURATION :
-
female 7-17 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Newborn - viability index (e.g., # alive at day 4 per # born alive)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Unreported
-
DOSE :
-
500 mg/kg
-
SEX/DURATION :
-
female 7-11 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - eye/ear Reproductive - Specific Developmental Abnormalities - urogenital system
MUTATION DATA
-
TYPE OF TEST :
-
Morphological transformation
-
TEST SYSTEM :
-
Rodent - rat Embryo
-
DOSE/DURATION :
-
1500 ng/plate
-
REFERENCE :
-
JJATDK JAT, Journal of Applied Toxicology. (John Wiley & Sons Ltd., Baffins Lane, Chichester, W. Sussex PO19 1UD, UK) V.1- 1981- Volume(issue)/page/year: 1,190,1981 *** REVIEWS *** IARC Cancer Review:Animal Sufficient Evidence IMEMDT IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Man. (WHO Publications Centre USA, 49 Sheridan Ave., Albany, NY 12210) V.1- 1972- Volume(issue)/page/year: 30,271,1983 IARC Cancer Review:Human No Adequate Data IMEMDT IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Man. (WHO Publications Centre USA, 49 Sheridan Ave., Albany, NY 12210) V.1- 1972- Volume(issue)/page/year: 30,271,1983 IARC Cancer Review:Group 2B IMSUDL IARC Monographs, Supplement. (WHO Publications Centre USA, 49 Sheridan Ave., Albany, NY 12210) No.1- 1979- Volume(issue)/page/year: 7,56,1987 TOXICOLOGY REVIEW APLMAS Archives of Pathology and Laboratory Medicine. (AMA, 535 N. Dearborn St., Chicago, IL 60610) V.1-5(2), 1926-28; V.100- 1976- Volume(issue)/page/year: 107,54,1983 *** OCCUPATIONAL EXPOSURE LIMITS *** OEL-FINLAND;Carcinogen JAN 1993 OEL-RUSSIA:STEL 1 mg/m3 JAN 1993
|