毒理学数据:
急性毒性LD50雄、雌小鼠,雄、雌大鼠(mg/kg):3224,2715,993,652口服;2158,1743,1655,2596腹腔注射;全部>5000皮下注射。急性毒性LD50大鼠致死量:>5000mg/kg。
LD50(mg/kg):雄小鼠,经口3224,腹腔注射2158,皮下注射>5000;雌小鼠,经口2715,腹腔注射1743,皮下
注射>5000;雄大鼠,经口993,腹腔注射1655,皮下注射>5000;雌大鼠,经口652,腹腔注射2596,皮下注射>5000.
CHEMICAL IDENTIFICATION
-
RTECS NUMBER :
-
NI3393500
-
CHEMICAL NAME :
-
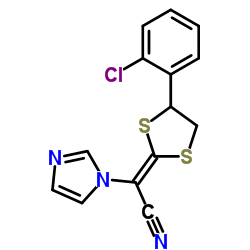
1H-Imidazole-1-acetonitrile, alpha-(4-(2-chlorophenyl)-1,3-dithiolan-2-ylidene)-, (E)-(+-)-
-
CAS REGISTRY NUMBER :
-
101530-10-3
-
LAST UPDATED :
-
199612
-
DATA ITEMS CITED :
-
17
-
MOLECULAR FORMULA :
-
C14-H10-Cl-N3-S2
-
MOLECULAR WEIGHT :
-
319.84
HEALTH HAZARD DATA
ACUTE TOXICITY DATA
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
652 mg/kg
-
TOXIC EFFECTS :
-
Autonomic Nervous System - other (direct) parasympathomimetic Lungs, Thorax, or Respiration - respiratory depression Skin and Appendages - hair
-
REFERENCE :
-
OYYAA2 Oyo Yakuri. Pharmacometrics. (Oyo Yakuri Kenkyukai, CPO Box 180, Sendai 980-91, Japan) V.1- 1967- Volume(issue)/page/year: 43,195,1992
-
TYPE OF TEST :
-
LD - Lethal dose
-
ROUTE OF EXPOSURE :
-
Administration onto the skin
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
>5 gm/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
OYYAA2 Oyo Yakuri. Pharmacometrics. (Oyo Yakuri Kenkyukai, CPO Box 180, Sendai 980-91, Japan) V.1- 1967- Volume(issue)/page/year: 43,195,1992
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
1655 mg/kg
-
TOXIC EFFECTS :
-
Autonomic Nervous System - other (direct) parasympathomimetic Behavioral - rigidity (including catalepsy) Behavioral - muscle contraction or spasticity
-
REFERENCE :
-
OYYAA2 Oyo Yakuri. Pharmacometrics. (Oyo Yakuri Kenkyukai, CPO Box 180, Sendai 980-91, Japan) V.1- 1967- Volume(issue)/page/year: 43,195,1992
-
TYPE OF TEST :
-
LD - Lethal dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
>5 gm/kg
-
TOXIC EFFECTS :
-
Behavioral - changes in motor activity (specific assay) Skin and Appendages - hair Nutritional and Gross Metabolic - weight loss or decreased weight gain
-
REFERENCE :
-
OYYAA2 Oyo Yakuri. Pharmacometrics. (Oyo Yakuri Kenkyukai, CPO Box 180, Sendai 980-91, Japan) V.1- 1967- Volume(issue)/page/year: 43,195,1992
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
2715 mg/kg
-
TOXIC EFFECTS :
-
Autonomic Nervous System - other (direct) parasympathomimetic Behavioral - somnolence (general depressed activity) Lungs, Thorax, or Respiration - respiratory depression
-
REFERENCE :
-
OYYAA2 Oyo Yakuri. Pharmacometrics. (Oyo Yakuri Kenkyukai, CPO Box 180, Sendai 980-91, Japan) V.1- 1967- Volume(issue)/page/year: 43,195,1992
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
1743 mg/kg
-
TOXIC EFFECTS :
-
Behavioral - somnolence (general depressed activity) Skin and Appendages - hair Nutritional and Gross Metabolic - body temperature decrease
-
REFERENCE :
-
OYYAA2 Oyo Yakuri. Pharmacometrics. (Oyo Yakuri Kenkyukai, CPO Box 180, Sendai 980-91, Japan) V.1- 1967- Volume(issue)/page/year: 43,195,1992
-
TYPE OF TEST :
-
LD - Lethal dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
>5 gm/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
OYYAA2 Oyo Yakuri. Pharmacometrics. (Oyo Yakuri Kenkyukai, CPO Box 180, Sendai 980-91, Japan) V.1- 1967- Volume(issue)/page/year: 43,195,1992
-
TYPE OF TEST :
-
LDLo - Lowest published lethal dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
SPECIES OBSERVED :
-
Mammal - dog
-
DOSE/DURATION :
-
2 gm/kg
-
TOXIC EFFECTS :
-
Behavioral - somnolence (general depressed activity) Liver - other changes Blood - normocytic anemia
-
REFERENCE :
-
OYYAA2 Oyo Yakuri. Pharmacometrics. (Oyo Yakuri Kenkyukai, CPO Box 180, Sendai 980-91, Japan) V.1- 1967- Volume(issue)/page/year: 43,137,1992 ** OTHER MULTIPLE DOSE TOXICITY DATA **
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
5040 mg/kg/4W-C
-
TOXIC EFFECTS :
-
Liver - other changes Endocrine - changes in spleen weight Skin and Appendages - dermatitis, other (after systemic exposure)
-
REFERENCE :
-
OYYAA2 Oyo Yakuri. Pharmacometrics. (Oyo Yakuri Kenkyukai, CPO Box 180, Sendai 980-91, Japan) V.1- 1967- Volume(issue)/page/year: 43,273,1992
-
TYPE OF TEST :
-
TCLo - Lowest published toxic concentration
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
4550 mg/kg/26W-I
-
TOXIC EFFECTS :
-
Liver - changes in liver weight Kidney, Ureter, Bladder - changes in bladder weight Nutritional and Gross Metabolic - weight loss or decreased weight gain
-
REFERENCE :
-
KSRNAM Kiso to Rinsho. Clinical Report. (Yubunsha Co., Ltd., 1-5, Kanda Suda-Cho, Chiyoda-ku, KS Bldg., Tokyo 101, Japan) V.1- 1960- Volume(issue)/page/year: 26,2439,1992
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Administration onto the skin
-
SPECIES OBSERVED :
-
Mammal - dog
-
DOSE/DURATION :
-
9100 mg/kg/26W-I
-
TOXIC EFFECTS :
-
Liver - other changes Biochemical - Enzyme inhibition, induction, or change in blood or tissue levels - phosphatases
-
REFERENCE :
-
KSRNAM Kiso to Rinsho. Clinical Report. (Yubunsha Co., Ltd., 1-5, Kanda Suda-Cho, Chiyoda-ku, KS Bldg., Tokyo 101, Japan) V.1- 1960- Volume(issue)/page/year: 26,2417,1992
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
SPECIES OBSERVED :
-
Mammal - dog
-
DOSE/DURATION :
-
700 mg/kg/4W-C
-
TOXIC EFFECTS :
-
Liver - changes in liver weight Blood - normocytic anemia Blood - changes in serum composition (e.g. TP, bilirubin, cholesterol)
-
REFERENCE :
-
OYYAA2 Oyo Yakuri. Pharmacometrics. (Oyo Yakuri Kenkyukai, CPO Box 180, Sendai 980-91, Japan) V.1- 1967- Volume(issue)/page/year: 43,137,1992 ** REPRODUCTIVE DATA **
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
DOSE :
-
7875 mg/kg
-
SEX/DURATION :
-
male 63 day(s) pre-mating
-
TOXIC EFFECTS :
-
Reproductive - Fertility - male fertility index (e.g. # males impregnating females per # males exposed to fertile nonpregnant females)
-
REFERENCE :
-
OYYAA2 Oyo Yakuri. Pharmacometrics. (Oyo Yakuri Kenkyukai, CPO Box 180, Sendai 980-91, Japan) V.1- 1967- Volume(issue)/page/year: 43,353,1992
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
DOSE :
-
550 mg/kg
-
SEX/DURATION :
-
female 7-17 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - post-implantation mortality (e.g. dead and/or resorbed implants per total number of implants) Reproductive - Specific Developmental Abnormalities - musculoskeletal system Reproductive - Effects on Newborn - stillbirth
-
REFERENCE :
-
OYYAA2 Oyo Yakuri. Pharmacometrics. (Oyo Yakuri Kenkyukai, CPO Box 180, Sendai 980-91, Japan) V.1- 1967- Volume(issue)/page/year: 43,369,1992
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
DOSE :
-
550 mg/kg
-
SEX/DURATION :
-
female 7-17 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Newborn - live birth index (measured after birth) Reproductive - Effects on Newborn - viability index (e.g., # alive at day 4 per # born alive) Reproductive - Effects on Newborn - behavioral
-
REFERENCE :
-
OYYAA2 Oyo Yakuri. Pharmacometrics. (Oyo Yakuri Kenkyukai, CPO Box 180, Sendai 980-91, Japan) V.1- 1967- Volume(issue)/page/year: 43,369,1992
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
DOSE :
-
2600 mg/kg
-
SEX/DURATION :
-
female 7-21 day(s) after conception lactating female 21 day(s) post-birth
-
TOXIC EFFECTS :
-
Reproductive - Effects on Embryo or Fetus - fetotoxicity (except death, e.g., stunted fetus)
-
REFERENCE :
-
OYYAA2 Oyo Yakuri. Pharmacometrics. (Oyo Yakuri Kenkyukai, CPO Box 180, Sendai 980-91, Japan) V.1- 1967- Volume(issue)/page/year: 43,383,1992
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
DOSE :
-
1300 mg/kg
-
SEX/DURATION :
-
female 6-13 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Maternal Effects - other effects
-
REFERENCE :
-
OYYAA2 Oyo Yakuri. Pharmacometrics. (Oyo Yakuri Kenkyukai, CPO Box 180, Sendai 980-91, Japan) V.1- 1967- Volume(issue)/page/year: 43,395,1992
|