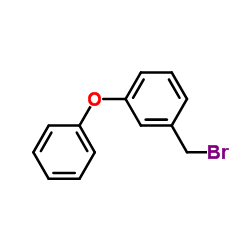
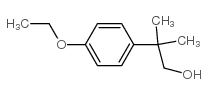
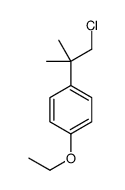
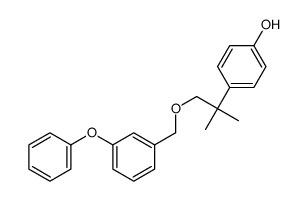
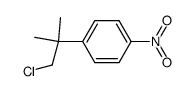
80844-07-1
| 中文名 | 醚菊酯 |
|---|---|
| 英文名 | etofenprox |
| 中文别名 |
多来宝
依芬普司 |
| 英文别名 |
TREBON
mti500 ETOPHENPROX Lenatop MFCD00210287 1-{[2-(4-Ethoxyphenyl)-2-methylpropoxy]methyl}-3-phenoxybenzene 1-[[2-(4-ethoxyphenyl)-2-methylpropoxy]methyl]-3-phenoxybenzene a-((p-ethoxy-b,b-dimethylphenethyl)oxy)-m-phenoxytoluene Ethophenprox PEPE Etofenprox NUKIL Ethofenprox PUNKASO 1-((2-(4-Ethoxyphenyl)-2-methylpropoxy)methyl)-3-phenoxybenzene EINECS 407-980-2 VECTRON Benzene, 1-[[2-(4-ethoxyphenyl)-2-methylpropoxy]methyl]-3-phenoxy- 2-(4-ethoxyphenyl)-2-methylpropyl 3-phenoxybenzyl ether |
| 密度 | 1.1±0.1 g/cm3 |
|---|---|
| 沸点 | 481.6±40.0 °C at 760 mmHg |
| 熔点 | 36ºC |
| 分子式 | C25H28O3 |
| 分子量 | 376.488 |
| 闪点 | 165.1±24.6 °C |
| 精确质量 | 376.203857 |
| PSA | 27.69000 |
| LogP | 7.34 |
| 外观性状 | 固体 |
| 蒸汽压 | 0.0±1.2 mmHg at 25°C |
| 折射率 | 1.559 |
| 储存条件 | 库房通风低温干燥0-6°C |
| 稳定性 | 化学性质稳定,于80℃贮存90d未见明显分解,在pH值2.8~11.9土壤中半衰期约6d。工业品溶点34~35℃。 |
| 计算化学 | 1.疏水参数计算参考值(XlogP):无 2.氢键供体数量:0 3.氢键受体数量:3 4.可旋转化学键数量:9 5.互变异构体数量:无 6.拓扑分子极性表面积27.7 7.重原子数量:28 8.表面电荷:0 9.复杂度:422 10.同位素原子数量:0 11.确定原子立构中心数量:0 12.不确定原子立构中心数量:0 13.确定化学键立构中心数量:0 14.不确定化学键立构中心数量:0 15.共价键单元数量:1 |
| 更多 | 1. 性状:白色晶体 2. 密度(g/mL25 ºC):未确定 3. 相对蒸汽密度(g/mL,空气=1):.157(23℃)。 4. 熔点(ºC):36.4~38 5. 沸点(ºC):208(719.8Pa)、100(3.2×10-2Pa) 6. 沸点(ºC1mmHg):未确定 7. 折射率:未确定 8. 闪点(ºC):未确定 9. 比旋光度(º):未确定 10. 自燃点或引燃温度(ºC):未确定 11. 蒸气压(Pa):32×10-3 (100℃)、8.0×10-3 (25℃), 12. 饱和蒸气压(kPa,60ºC):未确定 13. 燃烧热(KJ/mol):未确定 14. 临界温度(ºC):未确定 15. 临界压力(KPa):未确定 16. 油水(正辛醇/水)分配系数的对数值:11200000 17. 爆炸上限(%,V/V):未确定 18. 爆炸下(%,V/V):未确定 19. 溶解性:氯仿858g/L、丙酮908g/L、醋酸乙酯875g/L、乙醇150g/L、甲醇76.6g/L、二甲苯84.8g/L、水1mg/L |
|
毒理学数据: 口服- 大鼠 LD50: 42800 毫克 / 公斤; 口服- 小鼠 LD50: 107000 毫克/ 公斤 狗>5000mg/kg,LD50 > 1072mg /kg ,雌性>2140mg/kg;雄性小鼠急性经皮LD50 > 1072mg/kg, CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| 符号 |

GHS09 |
|---|---|
| 信号词 | Warning |
| 危害声明 | H362-H410 |
| 警示性声明 | P201-P260-P263-P273-P308 + P313-P391 |
| 个人防护装备 | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
| 危害码 (欧洲) | Xn |
| 危险品运输编码 | UN 3077 9 / PGIII |
| RTECS号 | DA0670000 |
|
~93% 
80844-07-1 |
| 文献:Galin, F. Z.; Rakhimov, R. G.; Tolstikov, G. A. Russian Journal of Organic Chemistry, 1993 , vol. 29, # 1.2 p. 170 Zhurnal Organicheskoi Khimii, 1993 , vol. 29, # 168 p. 202 - 203 |
|
~43% 
80844-07-1 |
| 文献:Russian Journal of Organic Chemistry, , vol. 38, # 11 p. 1629 - 1634 |
|
~% 
80844-07-1 |
| 文献:Russian Journal of Organic Chemistry, , vol. 38, # 11 p. 1629 - 1634 |
|
~% 
80844-07-1 |
| 文献:Russian Journal of Organic Chemistry, , vol. 38, # 11 p. 1629 - 1634 |
|
~% 
80844-07-1 |
| 文献:Russian Journal of Organic Chemistry, , vol. 38, # 11 p. 1629 - 1634 |
|
~% 
80844-07-1 |
| 文献:Russian Journal of Organic Chemistry, , vol. 38, # 11 p. 1629 - 1634 |
| 上游产品 7 | |
|---|---|
| 下游产品 0 | |