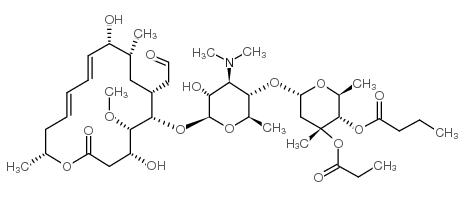
CHEMICAL IDENTIFICATION
-
RTECS NUMBER :
-
OH4726300
-
CHEMICAL NAME :
-
Leucomycin V, 4(sup B)-butanoate 3(Sup B)-propanoate
-
CAS REGISTRY NUMBER :
-
74014-51-0
-
LAST UPDATED :
-
199612
-
DATA ITEMS CITED :
-
9
-
MOLECULAR FORMULA :
-
C42-H69-N-O15
-
MOLECULAR WEIGHT :
-
828.12
HEALTH HAZARD DATA
ACUTE TOXICITY DATA
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
>4 gm/kg
-
TOXIC EFFECTS :
-
Gastrointestinal - other changes Nutritional and Gross Metabolic - weight loss or decreased weight gain
-
REFERENCE :
-
NKRZAZ Chemotherapy (Tokyo). (Nippon Kagaku Ryoho Gakkai, 2-20-8 Kamiosaki, Shinagawa-Ku, Tokyo 141, Japan) V.1- 1953- Volume(issue)/page/year: 32(Suppl 6),138,1984
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
>4 gm/kg
-
TOXIC EFFECTS :
-
Sense Organs and Special Senses (Olfaction) - effect, not otherwise specified Gastrointestinal - hypermotility, diarrhea
-
REFERENCE :
-
NKRZAZ Chemotherapy (Tokyo). (Nippon Kagaku Ryoho Gakkai, 2-20-8 Kamiosaki, Shinagawa-Ku, Tokyo 141, Japan) V.1- 1953- Volume(issue)/page/year: 32(Suppl 6),138,1984
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
>4 gm/kg
-
TOXIC EFFECTS :
-
Nutritional and Gross Metabolic - weight loss or decreased weight gain
-
REFERENCE :
-
NKRZAZ Chemotherapy (Tokyo). (Nippon Kagaku Ryoho Gakkai, 2-20-8 Kamiosaki, Shinagawa-Ku, Tokyo 141, Japan) V.1- 1953- Volume(issue)/page/year: 32(Suppl 6),138,1984
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
>5 gm/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
JANTAJ Journal of Antibiotics. (Japan Antibiotics Research Assoc., 2-20-8 Kamiosaki, Shinagawa-ku, Tokyo, 141, Japan) V.2-5, 1948-52; V.21- 1968- Volume(issue)/page/year: 34,1001,1981
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
>4 gm/kg
-
TOXIC EFFECTS :
-
Sense Organs and Special Senses (Olfaction) - effect, not otherwise specified Gastrointestinal - hypermotility, diarrhea
-
REFERENCE :
-
NKRZAZ Chemotherapy (Tokyo). (Nippon Kagaku Ryoho Gakkai, 2-20-8 Kamiosaki, Shinagawa-Ku, Tokyo 141, Japan) V.1- 1953- Volume(issue)/page/year: 32(Suppl 6),138,1984
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
>4 gm/kg
-
TOXIC EFFECTS :
-
Nutritional and Gross Metabolic - weight loss or decreased weight gain
-
REFERENCE :
-
NKRZAZ Chemotherapy (Tokyo). (Nippon Kagaku Ryoho Gakkai, 2-20-8 Kamiosaki, Shinagawa-Ku, Tokyo 141, Japan) V.1- 1953- Volume(issue)/page/year: 32(Suppl 6),138,1984
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Mammal - dog
-
DOSE/DURATION :
-
>360 mg/kg
-
TOXIC EFFECTS :
-
Gastrointestinal - nausea or vomiting
-
REFERENCE :
-
NKRZAZ Chemotherapy (Tokyo). (Nippon Kagaku Ryoho Gakkai, 2-20-8 Kamiosaki, Shinagawa-Ku, Tokyo 141, Japan) V.1- 1953- Volume(issue)/page/year: 32(Suppl 6),138,1984 ** OTHER MULTIPLE DOSE TOXICITY DATA **
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Mammal - dog
-
DOSE/DURATION :
-
65520 mg/kg/26W-I
-
TOXIC EFFECTS :
-
Gastrointestinal - changes in structure or function of salivary glands Gastrointestinal - nausea or vomiting Nutritional and Gross Metabolic - weight loss or decreased weight gain
-
REFERENCE :
-
JJANAX Japanese Journal of Antibiotics. (Japan Antibiotics Research Assoc., 2-20-8 Kamiosaki, Shinagawa-ku, Tokyo 141, Japan) V.21- 1968- Volume(issue)/page/year: 40,561,1987 ** REPRODUCTIVE DATA **
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
3900 mg/kg
-
SEX/DURATION :
-
female 6-18 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - litter size (e.g. # fetuses per litter; measured before birth)
-
REFERENCE :
-
JJANAX Japanese Journal of Antibiotics. (Japan Antibiotics Research Assoc., 2-20-8 Kamiosaki, Shinagawa-ku, Tokyo 141, Japan) V.21- 1968- Volume(issue)/page/year: 40,602,1987
|