异牡荆黄素
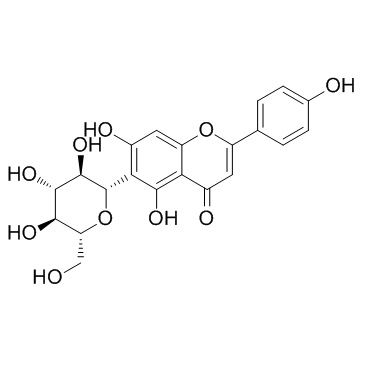
异牡荆黄素结构式

|
常用名 | 异牡荆黄素 | 英文名 | Isovitexin |
|---|---|---|---|---|
| CAS号 | 38953-85-4 | 分子量 | 432.378 | |
| 密度 | 1.7±0.1 g/cm3 | 沸点 | 807.0±65.0 °C at 760 mmHg | |
| 分子式 | C21H20O10 | 熔点 | 220 - 221 °C | |
| MSDS | 美版 | 闪点 | 287.1±27.8 °C |
异牡荆黄素用途Isovitexin 是从亚洲水稻种得到的黄酮类物质,具有抗氧化、抗炎的活性;Isovitexin 作用与 JNK1/2 的抑制剂类似,能够抑制 NF-κB 的活化。 |
| 中文名 | 异牡荆黄素 |
|---|---|
| 英文名 | Saponaretin |
| 英文别名 | 更多 |
| 描述 | Isovitexin 是从亚洲水稻种得到的黄酮类物质,具有抗氧化、抗炎的活性;Isovitexin 作用与 JNK1/2 的抑制剂类似,能够抑制 NF-κB 的活化。 |
|---|---|
| 相关类别 | |
| 靶点 |
JNK1 JNK2 NF-κB |
| 体外研究 | 异牡荆素通过抑制细胞内ROS的产生来预防LPS诱导的氧化损伤,并且还减弱H2O2对细胞活力的影响。含有LPS(2μg/ mL)的异牡荆素(0-100μg/ mL)对RAW 264.7细胞没有细胞毒性,但200μg/ mL异牡荆素显示出显着的细胞毒性。异牡荆素(25,50μg/ mL)抑制LPS诱导的TNF-α,IL-6,iNOS和COX-2水平的增加。异牡荆苷(25,50μg/ mL)也抑制RAW 264.7细胞中的IκBα磷酸化和降解,这种作用与JNK1/2抑制剂的作用一致[1]。 |
| 体内研究 | 异牡荆素(50和100mg/kg,ip)引起肺切片中较不严重的组织病理学变化,并减少LPS诱导的小鼠中的炎性细胞计数。异嗜酸蛋白(50和100 mg/kg,ip)通过降低TNF-α和IL-6产生,ROS产生,MPO和MDA含量,增加SOD和GSH,在LPS诱导的ALI小鼠中预防LPS诱导的炎症和氧化应激。水平并有效抑制iNOS和COX-2的蛋白表达[1]。异牡荆素(25,50,100mg/kg)剂量依赖性地降低小鼠中LPS/D-gal诱导的肝损伤的存活率。异牡荆素还抑制NF-κB的活化,并上调小鼠LPS/D-gal诱导的Nrf2和HO-1 [2]。 |
| 细胞实验 | 通过MTT测定确定细胞活力。将RAW 264.7细胞接种在96孔板(1×10 4个细胞/孔)中,并与各种浓度的异牡荆素(终浓度:0-200μg/ mL)和LPS(2μg/ mL)孵育24小时。此外,用IV(25或50μg/ mL)预处理细胞1小时,然后加入H 2 O 2(300μM)。 24小时后,将MTT(5mg / mL)加入细胞中,然后再孵育4小时[1]。 |
| 动物实验 | 小鼠[1]为了建立ALI模型,将小鼠随机分成6组:对照(盐水),仅异牡荆素(100 mg / kg,溶于0.5%DMSO),仅LPS(0.5 mg / kg,溶于盐水) ,LPS(0.5mg / kg)+异牡荆苷(50或100mg / kg)和LPS(0.5mg / kg)+地塞米松(Dex,5mg / kg溶于盐水中)。给予异牡荆苷或Dex(5mg / kg)异牡荆苷。在暴露于异牡荆素或Dex 1小时后,用乙醚麻醉小鼠,并鼻内(in)施用LPS以诱导肺损伤。 LPS施用12小时后,对动物实施安乐死。因此,收获支气管肺泡灌洗液(BALF)和肺组织样品以测量细胞因子水平; ROS生成; SOD,GSH,MDA和MPO活性;和COX-2,iNOS,HO-1和Nrf2蛋白的表达[1]。 |
| 参考文献 |
| 密度 | 1.7±0.1 g/cm3 |
|---|---|
| 沸点 | 807.0±65.0 °C at 760 mmHg |
| 熔点 | 220 - 221 °C |
| 分子式 | C21H20O10 |
| 分子量 | 432.378 |
| 闪点 | 287.1±27.8 °C |
| 精确质量 | 432.105652 |
| PSA | 181.05000 |
| LogP | 1.28 |
| 外观性状 | 淡黄粉末 |
| 蒸汽压 | 0.0±3.0 mmHg at 25°C |
| 折射率 | 1.743 |
| 储存条件 | ?20°C |
| 个人防护装备 | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| 危险品运输编码 | NONH for all modes of transport |
| WGK德国 | 3 |
| 海关编码 | 29329990 |
|
Activity-guided isolation, identification and quantification of biologically active isomeric compounds from folk medicinal plant Desmodium adscendens using high performance liquid chromatography with diode array detector, mass spectrometry and multidimentional nuclear magnetic resonance spectroscopy.
J. Pharm. Biomed. Anal. 102 , 54-63, (2014) The antioxidant activity of the crude extract (60% ethanol) from the leaves of Desmodium adscendens (Sw.) DC. (Fabaceae) was observed in DPPH, xanthine/xanthine oxidase, lipid peroxydation and neutrop... |
|
|
Identification and functional analysis of 2-hydroxyflavanone C-glucosyltransferase in soybean (Glycine max).
FEBS Lett. 589 , 1778-86, (2015) C-Glucosyltransferase is an enzyme that mediates carbon-carbon bond formation to generate C-glucoside metabolites. Although it has been identified in several plant species, the catalytic amino acid re... |
|
|
Simultaneous quantification of 25 active constituents in the total flavonoids extract from Herba Desmodii Styracifolii by high-performance liquid chromatography with electrospray ionization tandem mass spectrometry.
J. Sep. Sci. 38(7) , 1156-63, (2015) A sensitive and selective high-performance liquid chromatography coupled with electrospray ionization tandem mass spectrometry method has been developed and validated for the simultaneous determinatio... |
| Saponaretin |
| 4H-1-Benzopyran-4-one, 6-β-D-glucopyranosyl-5,7- dihydroxy-2-(4-hydroxyphenyl)- |
| Isovitexin |
| 6-b-D-Glucopyranosyl-5,7-dihydroxy-2-(4-hydroxyphenyl)-4H-1-benzopyran-4-one |
| D-Glucitol, 1,5-anhydro-1-C-[5,7-dihydroxy-2-(4-hydroxyphenyl)-4-oxo-4H-1-benzopyran-6-yl]-, (1S)- |
| 5,7-Dihydroxy-2-(4-hydroxyphenyl)-6-[(2S,3R,4R,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)tetrahydro-2H-pyran-2-yl]-4H-chromen-4-one |
| 5,7-Dihydroxy-2-(4-hydroxyphényl)-6-[(2S,3R,4R,5S,6R)-3,4,5-trihydroxy-6-(hydroxyméthyl)tétrahydro-2H-pyran-2-yl]-4H-chromén-4-one |
| (1S)-1,5-Anhydro-1-[5,7-dihydroxy-2-(4-hydroxyphenyl)-4-oxo-4H-chromen-6-yl]-D-glucitol |
| 5,7-Dihydroxy-2-(4-hydroxyphenyl)-6-[(2S,3R,4R,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)tetrahydro-2H-pyran-2-yl]-4H-chromen-4-on |
| Homovitexin |


