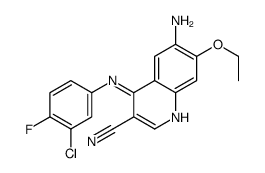
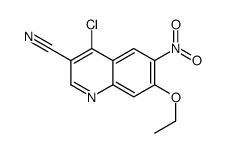
257933-82-7
| Name | pelitinib |
|---|---|
| Synonyms |
2-Butenamide,N-[4-[(3-chloro-4-fluorophenyl)amino]-3-cyano-7-ethoxy-6-quinolinyl]-4-(dimethylamino)-,(2E)
4-dimethylamino-but-2-enoic acid [4-(3-chloro-4-fluoro-phenylamino)-3-cyano-7-ethoxy-quinolin-6-yl]-amide 3-Cyano-4-[(3-chlor-4-fluorphenyl)amino]-6-{[4-(N,N-dimethylamino)-1-oxo-2-buten-1-yl]amino}-7-ethoxy-chinoline (2E)-N-[4-[(3-Chloro-4-fluorophenyl)amino]-3-cyano-7-ethoxy-6-quinolinyl]-4-(dimethylamino)-2-butenamide (2E)-N-{4-[(3-Chloro-4-fluorophenyl)amino]-3-cyano-7-ethoxyquinolin-6-yl}-4-(dimethylamino)but-2-enamide pelitinib (2E)-N-[4-[(3-chloro-4-fluorophenyl)amino]-3-cyano-7-ethoxy-6-quinolinyl]-4-(dimethylamino)-2-buteneamide WAY-EKB 569 EKB-569 (E)-N-(4-(3-chloro-4-fluorophenylamino)-3-cyano-7-ethoxyquinolin-6-yl)-4-(dimethylamino)but-2-enamide (2E)-N-{4-[(3-Chloro-4-fluorophenyl)amino]-3-cyano-7-ethoxy-6-quinolinyl}-4-(dimethylamino)-2-butenamide 2-Butenamide, N-[4-[(3-chloro-4-fluorophenyl)amino]-3-cyano-7-ethoxy-6-quinolinyl]-4-(dimethylamino)-, (2E)- 3-cyano-4-[(3-chloro-4-fluorophenyl)amino]-6-{[4-(N,N-dimethylamino)-1-oxo-2-buten-1-yl]amino}-7-ethoxy-quinoline |
| Description | Pelitinib (EKB-569;WAY-EKB 569) is an irreversible inhibitor of EGFR with an IC50 of 38.5 nM; also slightly inhibits Src, MEK/ERK and ErbB2 with IC50s of 282, 800, and 1255 nM, respectively. |
|---|---|
| Related Catalog | |
| Target |
EGFR:38.5 nM (IC50) |
| In Vitro | Pelitini has much greater inhibitory activity against the EGFR kinase than against Src, MEK/ERK, Cdk4, c-Met, Raf and ErbB2, for example, the IC50 for EGFR is 32-fold lower than the IC50 for the closely related ErbB2. Pelitinib results in a dramatic reduction in EGFR phosphorylation but no change in the total amount of EGFR protein. It requires at least 10-fold more drug to equivalently inhibit ErbB2 phosphorylation in similar assays, and EKB-569 does not block phosphorylation of another receptor tyrosine kinase (c-Met) assessed in the same manner[1]. EKB-569 is a potent inhibitor of proliferation in NHEK, A431, and MDA-468 cells (IC50=61, 125, and 260 nM, respectively) but not MCF-7 cells (IC50=3600 nM). EKB-569 is also a potent inhibitor of EGF-induced phosphorylated EGF-R (pEGF-R) in A431 and NHEK cells (IC50=20-80 nM)[1]. |
| In Vivo | A single oral dose of 10 mg/kg EKB-569 inhibits EGFR phosphorylation in A431 xenografts within 60 minutes. Twenty-four hours later, EGFR activity is still inhibited by over 50% by this single oral dose. The half-life of EKB-569 in mouse plasma is about 2 hours[1]. |
| Cell Assay | For experiments using cells in culture, A431 cells or 3T3/c-erbB-2 cells over-expressing c-erbB2 are are treated with various concentrations of EKB-569 for 2.75 h before co-incubation with 100 ng/mL EGF (A431 cells) or no growth factor (3T3/c-erbB-2 cells) for 0.25 h. Cells are ished twice with cold phosphate-buffered saline (PBS) before adding to lysis buffer for 20 min on ice, before immunoprecipitation and SDS–PAGE-immunoblotting[1]. |
| Animal Admin | Mice: For in vivo experiments, athymic nu/nu female mice are implanted subcutaneously with 5×106 A431 tumor cells. When tumors reach a mass of 200-300 mg, animals are treated with a single dose of 10 mg/kg EKB-569 in pH 2.0 water per gavage. Tumors from control and drug-treated animals are excised and minced into 1-mm pieces for anlysis[1]. |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 655.5±55.0 °C at 760 mmHg |
| Melting Point | 173-178ºC |
| Molecular Formula | C24H23ClFN5O2 |
| Molecular Weight | 467.923 |
| Flash Point | 350.2±31.5 °C |
| Exact Mass | 467.152435 |
| PSA | 90.28000 |
| LogP | 5.95 |
| Vapour Pressure | 0.0±2.0 mmHg at 25°C |
| Index of Refraction | 1.645 |
| Storage condition | -20°C Freezer |
| Water Solubility | DMSO: soluble5mg/mL, clear (warmed) |
| Symbol |

GHS06 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H301 |
| Precautionary Statements | P301 + P310 |
| Hazard Codes | T |
| Risk Phrases | 25 |
| Safety Phrases | 45 |
| RIDADR | UN 2811 6.1 / PGIII |
| Packaging Group | III |
| Precursor 9 | |
|---|---|
| DownStream 0 | |