54965-24-1
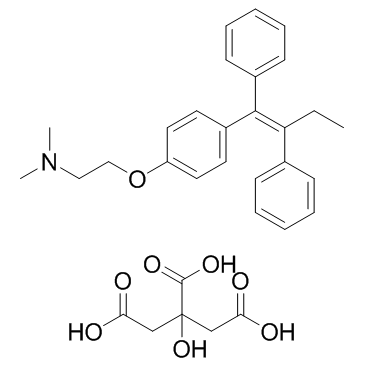
| Name | tamoxifen citrate |
|---|---|
| Synonyms |
trans-2-(p-(1,2-Diphenyl-1-butenyl)phenoxy)-N,N-dimethylethylamine citrate
Ethanamine, 2-[4-[(1Z)-1,2-diphenyl-1-buten-1-yl]phenoxy]-N,N-dimethyl-, 2-hydroxy-1,2,3-propanetricarboxylate (1:1) (salt) EINECS 259-415-2 2-{4-[(1Z)-1,2-Diphenylbut-1-en-1-yl]phenoxy}-N,N-dimethylethanamine 2-hydroxypropane-1,2,3-tricarboxylate (1:1) Tamoxifen, citrate salt MFCD00058321 Tamoxifen citrate salt 2YR&UYR&R DO2N1&1 &&Z Form citrate Tamoxifen citrate 2-{4-[(1Z)-1,2-Diphenyl-1-buten-1-yl]phenoxy}-N,N-dimethylethanamine 2-hydroxy-1,2,3-propanetricarboxylate (1:1) 2-{4-[(1Z)-1,2-Diphenylbut-1-en-1-yl]phenoxy}-N,N-dimethylethanaminium hydrogen 2-hydroxypropane-1,2,3-tricarboxylate (1:2:1) 2-{4-[(1Z)-1,2-diphenylbut-1-en-1-yl]phenoxy}-N,N-dimethylethanamine 2-hydroxypropane-1,2,3-tricarboxylate (salt) Tamoxifen (Citrate) |
| Description | Tamoxifen Citrate is a selective estrogen receptor modulator (SERM). |
|---|---|
| Related Catalog | |
| Target |
Estrogen receptor[1] |
| In Vitro | Tamoxifen shows strong inhibition of MCF-7 cells (EC50=1.41 μM) and to a lesser extent the T47D cells (EC50=2.5 μM) but does not affect the MDA-MB-231 cells[2]. |
| In Vivo | The Tamoxifen-inducible gene knockout strategy has clear advantages in that expression of a gene can be ablated in adult mice at will in a tissue specific manner. To study the role of Med1 in adult heart, 7-week old TmcsMed1-/- mice are given a daily Iintraperitoneal injection of Tamoxifen at a dose of 65 mg/kg for 5 days and killed at selected intervals thereafter. qPCR analysis of RNA shows that the Med1 expression begin to decrease after 3 days of Tamoxifen injection (about 70% decrease), and by 5 days of injection, Med1 expression is almost non-detectable in the heart. Tamoxifen-inducible cardiac-specific disruption of Med1 (TmcsMed1-/-) in adult mice causes dilated cardiomyopathy[3]. |
| Animal Admin | Mice[3] Seven-week old TmcsMed1-/- mice and the wild-type littermates are then administered Tamoxifen intraperitoneally at a daily dose of 65 mg/kg body weight for 5 days and then killed at selected intervals after initiation of Tamoxifen treatment. For each experiment 3 to 5 mice for control and csMed1-/- are used. To obtain survival curve 41 csMed1-/- and 41 csMed1fl/fl mice are used. Thirteen TmcsMed-/- mice and the same number of littermates are used for the survival curve experiments using Tamoxifen inducible model. The specific criteria for animal euthanasia included absence of food or water intake, slow or no mobility, weak or absence of heart beat, absence of palpitation of the chest as well as absence of respiratory movement. Mice are euthanized by intraperitoneal pentobarbital injection at the dose of 150mg/kg body weight to minimize suffering. |
| References |
[1]. Osborne CK. Tamoxifen in the treatment of breast cancer. N Engl J Med. 1998 Nov 26;339(22):1609-18. |
| Boiling Point | 665.9ºC at 760 mmHg |
|---|---|
| Melting Point | 140-144 °C |
| Molecular Formula | C32H37NO8 |
| Molecular Weight | 563.638 |
| Flash Point | 356.5ºC |
| Exact Mass | 563.251892 |
| PSA | 144.60000 |
| LogP | 4.74760 |
| Storage condition | 2-8°C |
| Water Solubility | slightly soluble |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Symbol |


GHS07, GHS08 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H302-H350-H360 |
| Precautionary Statements | P201-P280-P301 + P312 + P330-P308 + P313 |
| Personal Protective Equipment | Eyeshields;full-face particle respirator type N100 (US);Gloves;respirator cartridge type N100 (US);type P1 (EN143) respirator filter;type P3 (EN 143) respirator cartridges |
| Hazard Codes | T:Toxic |
| Risk Phrases | R22;R45 |
| Safety Phrases | S53-S36/37/39-S45 |
| RIDADR | UN 3077 9 / PGIII |
| WGK Germany | 3 |
| RTECS | KH2387000 |
| HS Code | 2922199090 |
|
~% 
54965-24-1 |
| Literature: Pharmaceutical Research, , vol. 23, # 4 p. 806 - 812 |
| Precursor 2 | |
|---|---|
| DownStream 0 | |
| HS Code | 2922199090 |
|---|---|
| Summary | 2922199090. other amino-alcohols, other than those containing more than one kind of oxygen function, their ethers and esters; salts thereof. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:30.0% |