217087-09-7
| Name | Esomeprazole Magnesium |
|---|---|
| Synonyms |
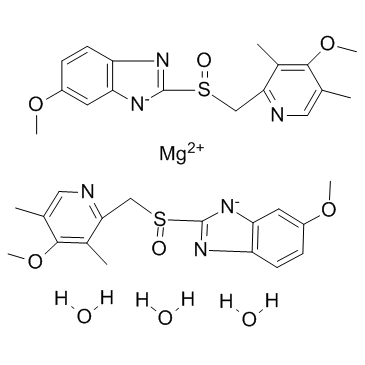
Esomeprazole magnesium trihydrate
1H-Benzimidazole, 5-methoxy-2-[[(4-methoxy-3,5-dimethyl-2-pyridinyl)methyl]sulfinyl]-, magnesium salt, hydrate (2:1:3) Esomeprazole magnesium EINECS 111-111-1 Magnesium 5-methoxy-2-{[(4-methoxy-3,5-dimethyl-2-pyridinyl)methyl]sulfinyl}benzimidazol-1-ide hydrate (1:2:3) Omeprazole magnesium trihydrate MFCD07698573 Magnesium 5-methoxy-2-{[(4-methoxy-3,5-dimethylpyridin-2-yl)methyl]sulfinyl}benzimidazol-1-ide hydrate (1:2:3) (S)-Omeprazole magnesium trihydrate Esomeprazole (Magnesium trihydrate) |
| Description | Esomeprazole Magnesium trihydrate is a proton pump inhibitor which reduces acid secretion through inhibition of the H+ / K+ ATPase in gastric parietal cells.IC50 value:Target: proton pumpEsomeprazole sodium (Nexium) is the S-isomer of omeprazole and acts as a proton pump inhibitor and gastric antisecretory agent indicated for the short-term treatment of gastroesophageal reflux disease in patients with a history of erosive esophagitis. Esomeprazole 0.4 and 0.8 mg/mL as the sodium salt in the infusion solutions tested is chemically and physically stable for at least 2 days at room temperature and 5 days under refrigeration. |
|---|---|
| Related Catalog | |
| References |
[4]. Esomeprazole |
| Boiling Point | 600ºC at 760 mmHg |
|---|---|
| Melting Point | 184-189ºC (dec.) |
| Molecular Formula | C34H42MgN6O9S2 |
| Molecular Weight | 767.167 |
| Flash Point | 316.7ºC |
| Exact Mass | 766.230530 |
| PSA | 198.59000 |
| LogP | 6.90950 |
| Vapour Pressure | 2.35E-14mmHg at 25°C |
| Storage condition | -20°C Freezer |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302 |
| Precautionary Statements | P301 + P312 + P330 |
| Hazard Codes | Xn |
| Risk Phrases | 22 |
| RIDADR | NONH for all modes of transport |