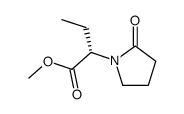
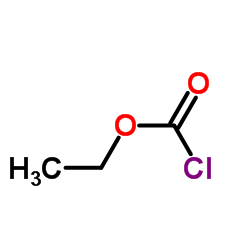
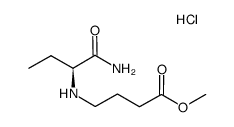
102767-28-2
| Name | levetiracetam |
|---|---|
| Synonyms |
(2S)-2-(2-Oxopyrrolidin-1-yl)butanamide
levetiracetamum 2(S)-(2-oxopyrrolidin-1-yl)butyramide KEPPRA (2S)-2-(2-Oxo-1-pyrrolidinyl)butanamide (S)-Etiracetam Etiracetam levo-isomer EINECS 200-659-6 ucb L059 (aS)-a-Ethyl-2-oxo-1-pyrrolidineacetamide 1-pyrrolidineacetamide, a-ethyl-2-oxo-, (aS)- 1-Pyrrolidineacetamide, α-ethyl-2-oxo-, (αS)- (S)-2-(2-Oxopyrrolidin-1-yl)Butanamide SIB S1 (S)-2-(2-Oxo-1-pyrrolidinyl)butyramide MFCD03265610 SIB-S1 (-)-(S)-α-Ethyl-2-oxo-1-pyrrolidineacetamide Keppra XR Levetiracetam |
| Description | Levetiracetam(UCB L059) is a novel anticonvulsant with antihyperalgesic efficacy in inflammatory pain.Target: Calcium ChannelLevetiracetam is used to control some types of seizures in patients with epilepsy. This medicine cannot cure epilepsy and will only work to control seizures for as long as you continue to use it. The exact mechanism for levetiracetam is unknown. However, the drug binds to a synaptic vesicle protein, SV2A, which is believed to impede nerve conduction across synapses [1].Levetiracetam (10-200 mg/kg), ibuprofen (12.5-100 mg/kg), celecoxib (3.75-30 mg/kg), paracetamol (50-200 mg/kg), caffeine (15-100 mg/kg), and 2-drug combinations of levetiracetam with analgesics/caffeine produced a significant, dose-dependent reduction of inflammatory hyperalgesia. Isobolographic analysis revealed that levetiracetam exerts a synergistic interaction with analgesics, with approximately 7-, 9-, and 11-fold reduction of doses of both drugs in combination of levetiracetam with paracetamol, celecoxib, and ibuprofen, respectively. Analysis of the log dose-response curves for levetiracetam (1-50 mg/kg) in the presence of caffeine (10 mg/kg) and levetiracetam applied alone also revealed a synergistic interaction. Levetiracetam's ED50 in the presence of caffeine was reduced approximately 11-fold [2].Clinical indications: Epilepsy; Social phobiaFDA Approved Date: November 2008Toxicity: depression; hallucinations; suicidal thoughts |
|---|---|
| Related Catalog | |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 395.9±25.0 °C at 760 mmHg |
| Melting Point | 118-119°C |
| Molecular Formula | C8H14N2O2 |
| Molecular Weight | 170.209 |
| Flash Point | 193.2±23.2 °C |
| Exact Mass | 170.105530 |
| PSA | 63.40000 |
| LogP | -0.67 |
| Vapour Pressure | 0.0±0.9 mmHg at 25°C |
| Index of Refraction | 1.519 |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302-H319 |
| Precautionary Statements | P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xn: Harmful; |
| Risk Phrases | R22 |
| Safety Phrases | S26 |
| RIDADR | NONH for all modes of transport |
| RTECS | UX9656166 |
| HS Code | 2933990090 |
| Precursor 8 | |
|---|---|
| DownStream 1 | |
| HS Code | 2933790090 |
|---|---|
| Summary | 2933790090. other lactams. VAT:17.0%. Tax rebate rate:9.0%. . MFN tariff:9.0%. General tariff:20.0% |