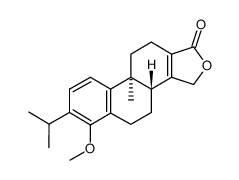
74285-86-2
| Name | 6-hydroxy-9b-methyl-7-propan-2-yl-3,3b,4,5,10,11-hexahydronaphtho[2,1-e][2]benzofuran-1-one |
|---|---|
| Synonyms |
Triptophenolide
Phenanthro[1,2-c]furan-1(3H)-one, 3b,4,5,9b,10,11-hexahydro-6-hydroxy-9b-methyl-7-(1-methylethyl)-, (3bR,9bS)- 6-hydroxy-9b-methyl-7-propan-2-yl-3,3b,4,5,10,11-hexahydronaphtho[2,1-e]isobenzofuran-1-one (3bR,9bS)-6-Hydroxy-7-isopropyl-9b-methyl-3b,4,5,9b,10,11-hexahydrophenanthro[1,2-c]furan-1(3H)-one |
| Description | Triptophenolide is a colorless crystalline plate isolated from ethyl acetate extracts of Tripterygium wilfordii. IC50 value:Target:In vitro: Triptophenolide can remarkably inhibit the delayed type hypersensitivity (DTH) reaction induced by DNCB and BSA; and diminished the peripheral blood ANAE+lymphocytes in rats and micc. Moreover, triptophenolide can dramatically increase the amount of total serum complement and significautly decrcase the serum antibody products (1gG ) of rats and mice. The phagocytosis of perioneal exudate macrophages in mice present double effects in vitro [1].In vivo: |
|---|---|
| Related Catalog | |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 490.0±45.0 °C at 760 mmHg |
| Melting Point | 232-233ºC |
| Molecular Formula | C20H24O3 |
| Molecular Weight | 312.403 |
| Flash Point | 202.4±21.5 °C |
| Exact Mass | 312.172546 |
| PSA | 46.53000 |
| LogP | 3.92 |
| Vapour Pressure | 0.0±1.3 mmHg at 25°C |
| Index of Refraction | 1.603 |
| Storage condition | -20°C |
|
~10% 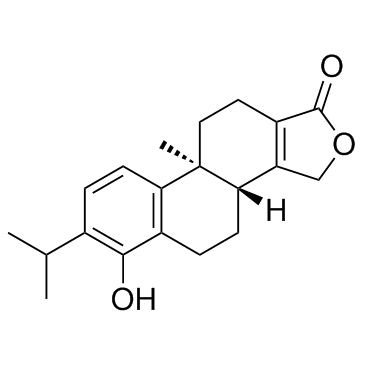
74285-86-2 |
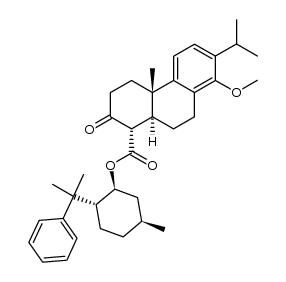
| Literature: Journal of Organic Chemistry, , vol. 65, # 7 p. 2208 - 2217 |
|
~% 
74285-86-2 |
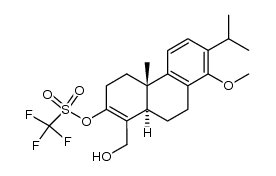
| Literature: Journal of Organic Chemistry, , vol. 65, # 7 p. 2208 - 2217 |
|
~% 
74285-86-2 |
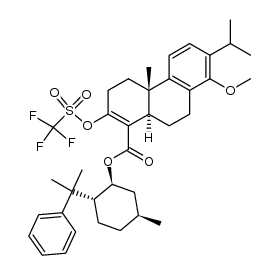
| Literature: Journal of Organic Chemistry, , vol. 65, # 7 p. 2208 - 2217 |
|
~% 
74285-86-2 |
| Literature: Journal of Organic Chemistry, , vol. 65, # 7 p. 2208 - 2217 |
| Precursor 4 | |
|---|---|
| DownStream 0 | |