1999-42-4
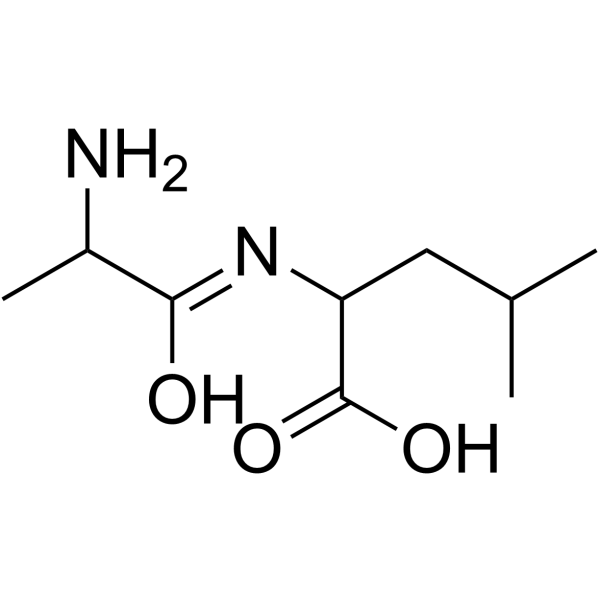
| Name | 2-(2-aminopropanoylamino)-4-methylpentanoic acid |
|---|---|
| Synonyms |
(+-)-N-Alanyl-leucin
D,L-alanyl-D,L-leucine (+-)-N-alanyl-leucine alanylleucine opt.-inakt. N-Alanyl-leucin (S,S)2-(2-amino-propionylamino)-4-methyl-pentanoic acid DL-ALA-DL-LEU DL-Alanyl-DL-Lencine D-Ala-D-Leu-OH/D-Ala-L-Leu-OH/L-Ala-D-Leu-OH/L-Ala-L-Leu-OH,(1:1:1:1) DL-ALANINE-DL-LEUCINE DL-Alanyl-DL-leucine H-DL-ALA-DL-LEU-OH DL-Alanyl-DL-leucin MFCD00025555 |
| Description | 2-(2-Aminopropanamido)-4-methylpentanoic acid is a leucine derivative[1]. |
|---|---|
| Related Catalog | |
| In Vitro | Amino acids and amino acid derivatives have been commercially used as ergogenic supplements. They influence the secretion of anabolic hormones, supply of fuel during exercise, mental performance during stress related tasks and prevent exercise induced muscle damage. They are recognized to be beneficial as ergogenic dietary substances[1]. |
| References |
| Density | 1.108 g/cm3 |
|---|---|
| Boiling Point | 409.7ºC at 760 mmHg |
| Molecular Formula | C9H18N2O3 |
| Molecular Weight | 202.25100 |
| Flash Point | 201.6ºC |
| Exact Mass | 202.13200 |
| PSA | 92.42000 |
| LogP | 1.04030 |
| Storage condition | −20°C |
| WGK Germany | 3 |
|---|
|
~% 
1999-42-4 |
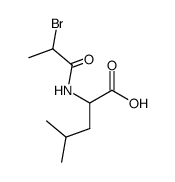
| Literature: Justus Liebigs Annalen der Chemie, , vol. 340, p. 171 |
|
~% 
1999-42-4 |
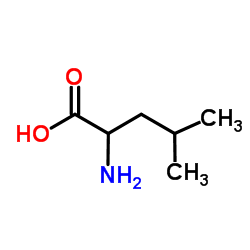
| Literature: Justus Liebigs Annalen der Chemie, , vol. 340, p. 171 |
|
~% 
1999-42-4 |
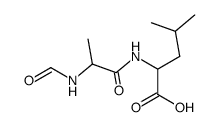
| Literature: Justus Liebigs Annalen der Chemie, , vol. 636, p. 140 - 143 |
|
~% 
1999-42-4 |
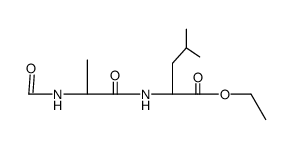
| Literature: Justus Liebigs Annalen der Chemie, , vol. 636, p. 140 - 143 |
|
~% 
1999-42-4 |
| Literature: Justus Liebigs Annalen der Chemie, , vol. 636, p. 140 - 143 |
|
~% 
1999-42-4 |
| Literature: Hoppe-Seyler's Zeitschrift fuer Physiologische Chemie, , vol. 154, p. 223 |
| Precursor 4 | |
|---|---|
| DownStream 1 | |