24751-69-7
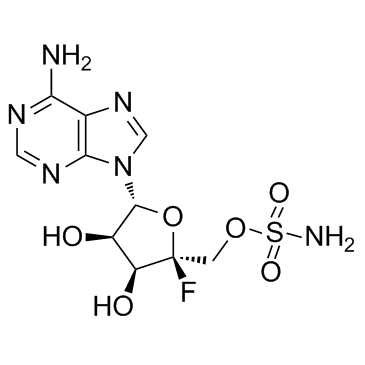
| Name | 4'-Fluoro-5'-O-sulfamoyladenosine |
|---|---|
| Synonyms |
T-3018
Antibiotic T-3018 4'-Fluor-O5'-sulfamoyl-adenosin 5'-O-Sulfamoyl-4'-fluoroadenosine 4'-Fluor-5'-O-sulfamoyladenosin nucleocidin Sulfamic acid 4'-fluoro-5'-adenosyl ester 4'-fluoro-O5'-sulfamoyl-adenosine |
| Description | Nucleocidin is an antitrypanosomal antibiotic, inhibiting the transfer of labeled amino acid from S-RNA to protein. |
|---|---|
| Related Catalog | |
| Target |
Antibacterial[1]. |
| In Vitro | Although appreciable inhibition occurrs at low concentrations of nucleocidin, it is not possible to obtain complete inhibition even at 10-3 M. An appreciable lag occurrs before any inhibition by nucleocidin is detectable, and the length of this lag period varies inversely with the concentration of nucleocidin. Similar results are obtained when nucleocidin is added to the cell-free system from reticulocytes. By contrast, essentially complete inhibition is obtained at 10-3 M puromycin, and only at the lowest concentrations is any lag detectable. It seems that nucleocidin exhibits the pattern typical of many antibiotics, i.e. it inhibits incorporation at a stage subsequent to the formation of aminoacyl-S-RNA[1]. |
| References |
| Density | 2.23g/cm3 |
|---|---|
| Boiling Point | 747.6ºC at 760mmHg |
| Molecular Formula | C10H13FN6O6S |
| Molecular Weight | 364.31000 |
| Flash Point | 405.9ºC |
| Exact Mass | 364.06000 |
| PSA | 197.08000 |
| Vapour Pressure | 1.79E-23mmHg at 25°C |
| Index of Refraction | 1.848 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|