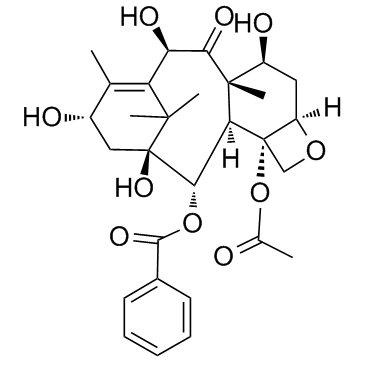
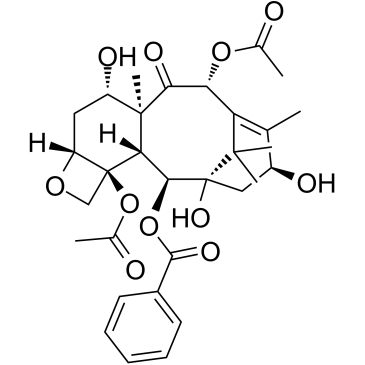
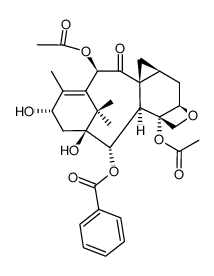
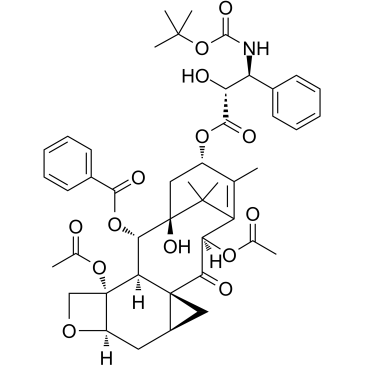
156294-36-9
| Name | (3ξ,5β,7β,10β)-4,10-Diacetoxy-1-hydroxy-13-{[(3S)-2-hydroxy-3-({[ (2-methyl-2-propanyl)oxy]carbonyl}amino)-3-phenylpropanoyl]oxy}-9 -oxo-5,20-epoxy-7,19-cyclotax-11-en-2-yl benzoate |
|---|---|
| Synonyms |
acetylcyclopropyl docetaxel
Valiolamine hydrate Benzenepropanoic acid, β-[[(1,1-dimethylethoxy)carbonyl]amino]-α-hydroxy-, (1S,2S,4S,7R,8aR,9aS,10aR,12aS,12bR)-7,12a-bis(acetyloxy)-1-(benzoyloxy)-1,3,4,7,8,9,9a,10,10a,12,12a,12b-dodecahydro- 2-hydroxy-5,13,13-trimethyl-8-oxo-2,6-methano-2H-cyclodeca[3,4]cyclopropa[4,5]benz[1,2-b]oxet-4-yl ester, (αR,βS)- Valinolamine valiolamine acetocyclopropyl taxotere (2α,5β,7β,10β,13α)-4,10-Diacetoxy-1-hydroxy-13-{[(2R,3S)-2-hydroxy-3-({[(2-methyl-2-propanyl)oxy]carbonyl}amino)-3-phenylpropanoyl]oxy}-9-oxo-5,20-epoxy-7,19-cyclotax-11-en-2-yl benzo 
ate |
| Description | Larotaxel (XRP9881) is a taxane analogue with preclinical activity against taxane-resistant breast cancer. Larotaxel (XRP9881) exerts its cytotoxic effect by promoting tubulin assembly and stabilizing microtubules, ultimately leading to cell death by apoptosis. It presents the ability to cross the blood brain barrier and has a much lower affinity for P-glycoprotein 1 than Docetaxel[1][2][3]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 889.3±65.0 °C at 760 mmHg |
| Molecular Formula | C45H53NO14 |
| Molecular Weight | 831.901 |
| Flash Point | 491.7±34.3 °C |
| Exact Mass | 831.346619 |
| PSA | 213.78000 |
| LogP | 7.06 |
| Vapour Pressure | 0.0±0.3 mmHg at 25°C |
| Index of Refraction | 1.613 |
|
~% 
156294-36-9 |
| Literature: US2012/149925 A1, ; Page/Page column 17 ; |
|
~% 
156294-36-9 |
| Literature: US2012/149925 A1, ; |
|
~% 
156294-36-9 |
| Literature: US2012/149925 A1, ; |
|
~% 
156294-36-9 |
| Literature: US2012/149925 A1, ; |
| Precursor 3 | |
|---|---|
| DownStream 0 | |