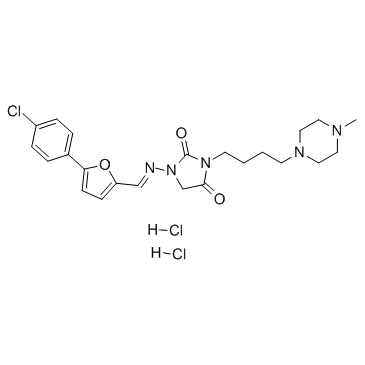
149888-94-8
| Name | 1-[(E)-[5-(4-chlorophenyl)furan-2-yl]methylideneamino]-3-[4-(4-methylpiperazin-1-yl)butyl]imidazolidine-2,4-dione,dihydrochloride |
|---|---|
| Synonyms |
azmilide
Azimilide hydrochloride Stedicor Azimilide HCl Azimilide dihydrochloride Azimilide (Dihydrochloride) |
| Description | Azimilide 2Hcl(NE-10064 2Hcl) is a class III antiarrhythmic compound, inhibits I(Ks) and I(Kr) in guinea-pig cardiac myocytes and I(Ks) (minK) channels expressed in Xenopus oocytes.IC50 value:Target: in vitro: Azimilide blocked HERG channels at 0.1 and 1 Hz with IC50s of 1.4 microM and 5.2 microM respectively. Azimilide blockade of HERG channels expressed in Xenopus oocytes and I(Kr) in mouse AT-1 cells was decreased under conditions of high [K+]e, whereas block of slowly activating I(Ks) channels was not affected by changes in [K+]e [1]. Azimilide suppressed the following currents (Kd in parenthesis): IKr (< 1 microM at -20 mV), IKs (1.8 microM at +30 mV), L-type Ca current (17.8 microM at +10 mV), and Na current (19 microM at -40 mV). Azimilide was a weak blocker of the transient outward and inward rectifier currents (Kd > or = 50 microM at +50 and -140 mV, respectively). Azimilide blocked IKr, IKs, and INa in a use-dependent manner. Furthermore, azimilide reduced a slowly inactivating component of Na current that might be important for maintaining the action potential plateau in canine ventricular myocytes [2]. In guinea pig ventricular myocytes, NE-10064 (0.3-3 microM) significantly prolonged action potential duration (APD) at 1 Hz. At 3 Hz, NE-10064 (0.3-1 microM) increased APD only slightly, and at 10 microM decreased APD and the plateau potential. NE-10064 potently blocked the rapidly activating component of the delayed rectifier, IKr (IC50 0.4 microM), and inhibited IKs (IC50 3 microM) with nearly 10-fold less potency [3].in vivo: NE-10064 (10 mg/kg intravenously, i.v.) reduced (p < 0.05) the incidence (8 of 12) of PES-induced ventricular tachycardia (VT). The cycle length of induced VT was not prolonged by NE-10064 (0.245 +/- 0.046 s predrug vs. 0.301 +/- 0.060 s postdrug). NE-10064 increased ventricular effective refractory period (VERP 166 +/- 5 ms predrug vs. 194 +/- 13 ms postdrug, p = 0.013), prolonged QTc interval (310 +/- 12 ms predrug vs. 350 +/- 16 ms postdrug, p = 0.004) and prolonged the effective refractory period (ERP) of noninfarcted myocardium (p = 0.045) [4]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.32g/cm3 |
|---|---|
| Boiling Point | 594.9ºC at 760mmHg |
| Molecular Formula | C23H30Cl3N5O3 |
| Molecular Weight | 530.87500 |
| Flash Point | 313.6ºC |
| Exact Mass | 529.14100 |
| PSA | 72.60000 |
| LogP | 4.58130 |
| Storage condition | 2-8℃ |
| Symbol |

GHS06 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H301 |
| Precautionary Statements | P301 + P310 |
| RIDADR | UN 2811 6.1 / PGIII |