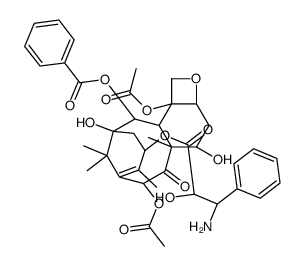
153415-45-3
| Name | Taxol C |
|---|---|
| Synonyms |
(2α,5β,7β,10β,13α)-4,10-bis(acetyloxy)-13-{[(2R,3S)-3-(hexanoylamino)-2-hydroxy-3-phenylpropanoyl]oxy}-1,7-dihydroxy-9-oxo-5,20-epoxytax-11-en-2-yl benzoate
Benzenepropanoic acid, α-hydroxy-β-[(1-oxohexyl)amino]-, (2aR,4S,4aS,6R,9S,11S,12S,12aR,12bS)-6,12b-bis(acetyloxy)-12-(benzoyloxy)-2a,3,4,4a,5,6,9,10,11,12,12a,12b-dodecahydro-4,11-dihydroxy-4a ,8,13,13-tetramethyl-5-oxo-7,11-methano-1H-cyclodeca[3,4]benz[1,2-b]oxet-9-yl ester, (αR,βS)- N-Debenzoyl-N-hexanoylpaclitaxel (-)-Taxuyunnanine taxuyunnansine benzenepropanoic acid, α-hydroxy-β-[(1-oxohexyl)amino]-, (2aR,4S,4aS,6R,9S,11S,12S,12aR,12bS)-6,12b-bis(acetyloxy)-12-(benzoyloxy)-2a,3,4,4a,5,6,9,10,11,12,12a,12b-dodecahydro-4,11-dihydroxy-4a,8,13,13-tetramethyl-5-oxo-7,11-methano-1H-cyclodeca[3,4]benz[1,2-b]oxet-9-yl ester, (αR,βS)- Paclitaxel C Paclitaxel impurity C taxuyunnanin (2α,5β,7β,10β,13α)-4,10-Diacetoxy-13-{[(2R,3S)-3-(hexanoylamino)-2-hydroxy-3-phenylpropanoyl]oxy}-1,7-dihydroxy-9-oxo-5,20-epoxytax-11-en-2-yl benzoate Taxuyunnanine Taxuyunnanine A |
| Description | Paclitaxel C is a compound that can be found in taxus cutting[1]. |
|---|---|
| Related Catalog | |
| References |
[1]. Rene SCHEPENS, et al. Production of taxol from taxus cuttings. WO2022162101A1. |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 932.3±65.0 °C at 760 mmHg |
| Molecular Formula | C46H57NO14 |
| Molecular Weight | 847.94 |
| Flash Point | 517.6±34.3 °C |
| Exact Mass | 847.377930 |
| PSA | 224.78000 |
| LogP | 7.22 |
| Vapour Pressure | 0.0±0.3 mmHg at 25°C |
| Index of Refraction | 1.604 |
|
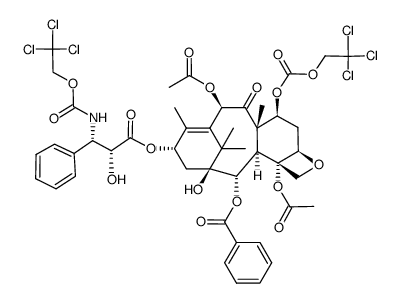
~57% 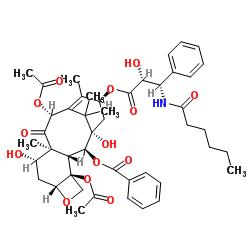
153415-45-3 |
| Literature: Skwarczynski, Mariusz; Sohma, Youhei; Noguchi, Mayo; Kimura, Maiko; Hayashi, Yoshio; Hamada, Yoshio; Kimura, Tooru; Kiso, Yoshiaki Journal of Medicinal Chemistry, 2005 , vol. 48, # 7 p. 2655 - 2666 |
|
~% 
153415-45-3 |
| Literature: Journal of Medicinal Chemistry, , vol. 48, # 7 p. 2655 - 2666 |
| Precursor 2 | |
|---|---|
| DownStream 0 | |