155141-29-0
| Name | rosiglitazone maleate |
|---|---|
| Synonyms |
(5-(4-(2-(N-Methyl-N-(2-pyridinyl)amino)ethoxy)benzylidene)-2,4-thiazolidinedione
(5E)-5-(4-{2-[Methyl(pyridin-2-yl)amino]ethoxy}benzylidene)-1,3-thiazolidine-2,4-dione 5-[[4-[2-(Methyl-2-pyridinylamino)ethoxy]phenyl]methyl]-2,4-thiazolidinedione maleate salt 2,4-Thiazolidinedione, 5-[[4-[2-(methyl-2-pyridinylamino)ethoxy]phenyl]methylene]-, (5E)- MFCD03427306 (5E)-5-(4-{2-[Methyl(2-pyridinyl)amino]ethoxy}benzylidene)-1,3-thiazolidine-2,4-dione Rosiglitazone maleate Rosiglitazone (maleate) |
| Description | Rosiglitazone maleate is a potent and selective activator of PPARγ, with EC50s of 30 nM, 100 nM and 60 nM for PPARγ1, PPARγ2, and PPARγ, respectively, and a Kd of appr 40 nM for PPARγ; Rosiglitazone maleate is also an modulator of TRP channels, inhibits TRP melastatin 2 (TRPM2), TRPM3 and activates TRP canonical 5 (TRPC5). |
|---|---|
| Related Catalog | |
| Target |
PPARγ1:30 nM (EC50) PPARγ2:100 nM (EC50) |
| In Vitro | Rosiglitazone maleate is a potent and selective activator of PPARγ, with EC50s of 30 nM and 100 nM for PPARγ1 and PPARγ2, respectively, and a Kd of appr 40 nM for PPARγ. Rosiglitazone (BRL49653, 0.1, 1,10 μM) promotes differentiation of C3H10T1/2 stem cells to adipocytes[1]. Rosiglitazone (Compound 6) activates PPARγ, with an EC50 of 60 nM[2]. Rosiglitazone (1 μM) activates PPARγ, which binds to NF-α1 promoter to activate gene transcription in neurons. Rosiglitazone (1 μM) also protects Neuro2A cells and hippocampal neurons against oxidative stress, and up-regulates BCL-2 expression in an NF-α1-dependent manner[3]. Rosiglitazone completely inhibits TRPM3 with IC50 values of 9.5 and 4.6 μM against nifedipine- and PregS-evoked activity, but such effects are not via PPARγ. Rosiglitazone inhibits TRPM2 at higher concentration, with an IC50 of appr 22.5 μM. Rosiglitazone is a strong stimulator of TRPC5 channels, with an EC50 of ∼30 μM[4]. |
| In Vivo | Rosiglitazone (5 mg/kg, p.o.) decreases the serum glucose in diabetic rats. Rosiglitazone also decreases IL-6, TNF-α, and VCAM-1 levels in diabetic group. Rosiglitazone in combination with losartan increases glucose compared to diabetic and Los-treated groups. Rosiglitazone significantly ameliorates endothelial dysfunction indicated by a significantly lower contractile response to PE and Ang II and enhancement of ACh-provoked relaxation in aortas isolated from diabetic rats[5]. |
| Kinase Assay | cDNA encoding amino acids 174-475 of PPARγ1 is amplified via polymerase chain reaction and inserted into bacterial expression vector pGEX-2T. GST-PPARγ LBD is expressed in BL21(DE3)plysS cells and extracts. For saturation binding analysis, bacterial extracts (100 μg of protein) are incubated at 4°C for 3 h in buffer containing 10 mM Tris (pH 8.0), 50 mM KCl, 10 mM dithiothreitol with [3H]-BRL49653 (specific activity, 40 Ci/mmol) in the presence or absence of unlabeled Rosiglitazone. Bound is separated from free radioactivity by elution through 1-mL Sephadex G-25 desalting columns. Bound radioactivity eluted in the column void volume and is quantitated by liquid scintillation counting[1]. |
| Cell Assay | C3H10T1/2 cells are grown in a 24-well plate in DME medium supplemented with 10% fetal calf serum. Medium and compound (Rosiglitazone) are exchanged every 3 days. Cells are stained at day 7 with Oil Red O and photographed[1]. |
| Animal Admin | Rats are intravenously injected with 38 mg/kg streptozotocin and after 48 h, diabetes is identified by urinary glucosuria and then random blood sugar is measured and this day is regarded as day 0. Animals with a serum glucose level of 220-300 mg/dL are selected to be used in this study. Rats are randomly separated into five groups for daily drug administration for 8 weeks: group 1: control nondiabetic rats given a vehicle only (0.5 mL/kg of 0.5% carboxy methyl celleluse orally), group 2: control diabetic rats given a vehicle, group 3: diabetic rats receiving Rosiglitazone (5 mg/kg orally), group 4: diabetic rats receiving losartan (2 mg/kg, orally), and group 5: diabetic rats receiving both Rosiglitazone and losartan[2]. |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 585ºC at 760 mmHg |
| Melting Point | 235-240°C |
| Molecular Formula | C22H23N3O7S |
| Molecular Weight | 473.50 |
| Flash Point | 307.6ºC |
| PSA | 96.83000 |
| LogP | 2.38 |
| Index of Refraction | 1.688 |
| Storage condition | -20°C Freezer |
|
~97% 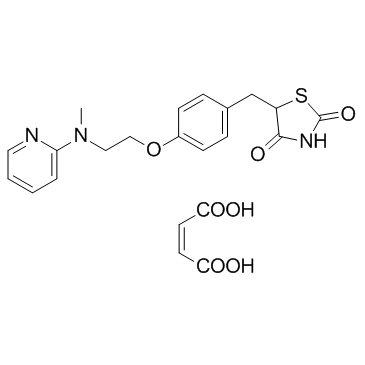
155141-29-0 |
| Literature: Lopez, Ernesto Duran Patent: US2009/234128 A1, 2009 ; Location in patent: Page/Page column 5-6 ; |
| Precursor 2 | |
|---|---|
| DownStream 0 | |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |