13209-41-1
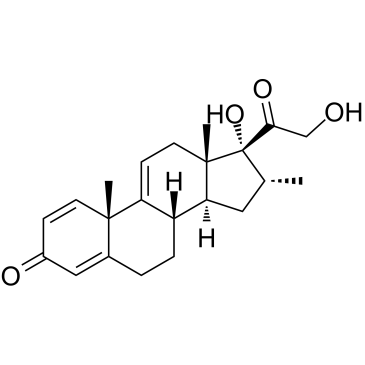
| Name | 16α-Methyl-9,11-dehydro Prednisolone |
|---|---|
| Synonyms | (8S,10S,13S,14S,16R,17R)-17-hydroxy-17-(2-hydroxyacetyl)-10,13,16-trimethyl-7,8,12,14,15,16-hexahydro-6H-cyclopenta[a]phenanthren-3-one |
| Description | Vamorolone (VBP15) is a first-in-class, orally active dissociative steroidal anti-inflammatory drug and membrane-stabilizer. Vamorolone improves muscular dystrophy without side effects. Vamorolone shows potent NF-κB inhibition and substantially reduces hormonal effects[1][2]. |
|---|---|
| Related Catalog | |
| In Vitro | Vamorolone (VBP15) inhibits TNFα-induced pro-inflammatory NF-κB signaling in C2C12 muscle cells at 1 nM or more. Vamorolone binds the glucocorticoid receptor (GR) and mineralocorticoid receptor (MR) with similar affinity[1]. Vamorolone (0.1, 1μM; 30 minutes) reduces production of IL1βand CCL5 inflammatory mediators in primary human macrophages[2]. Vamorolone is a first-in-class mineralocorticoid receptor (MR) antagonist/dissociative glucocorticoid receptor (GR) ligand[3]. |
| In Vivo | Vamorolone (5-30 mg/kg; cherry syrup) shows a superior side effect profile compared to pharmacological glucocorticoids in mdx mice[1]. Vamorolone (30 mg/kg; orally; daily for 20 days) reduces CNS Inflammation in murine experimental autoimmune encephalomyelitis[2]. Animal Model: C57BL/6 mice (experimental autoimmune encephalomyelitis)[2] Dosage: 30 mg/kg Administration: Orally; daily for 20 days (starting one day prior to MOG 33-55 peptide immunization and continuing) Result: Reduced CNS inflammation in murine experimental autoimmune encephalomyelitis. |
| References |
| Density | 1.24g/cm3 |
|---|---|
| Boiling Point | 548.3ºC at 760 mmHg |
| Molecular Formula | C22H28O4 |
| Molecular Weight | 356.45500 |
| Flash Point | 299.4ºC |
| Exact Mass | 356.19900 |
| PSA | 74.60000 |
| LogP | 2.75290 |
| Vapour Pressure | 2.63E-14mmHg at 25°C |
| Index of Refraction | 1.603 |
| Precursor 0 | |
|---|---|
| DownStream 1 | |