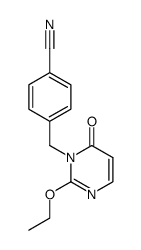
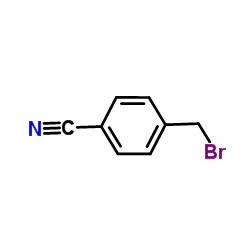
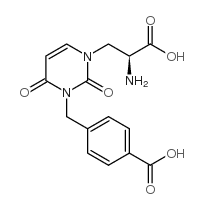
544697-47-4
| Name | UBP 282,(αS)-α-Amino-3-[(4-carboxyphenyl)methyl]-3,4-dihydro-2,4-dioxo-1(2H)-pyrimidinepropanoicacid |
|---|---|
| Synonyms | ubp 282 |
| Description | UBP-282 is a potent, selective and competitive AMPA and kainate receptor antagonist. UBP-282 inhibits the fast component of the dorsal root-evoked ventral root potential (fDR-VRP) with an IC50 value of 10.3 μM. UBP-282 antagonizes kainate-induced depolarisations of dorsal roots with a pA2 value of 4.96[1][2]. |
|---|---|
| Related Catalog | |
| Target |
IC50: 10.3 μM (fast component of the dorsal root-evoked ventral root potential (fDR-VRP))[1] pA2: 4.96 (Kainate<-induced depolarisations of dorsal roots)[1] |
| In Vitro | UBP-282 (3-CBW) is selective for AMPA- and GluR5-containing kainate receptors vs NMDA, mGlu and kainate receptors expressed on motor neurones[1][2]. UBP-282 (3-CBW), at a concentration of 200 μM, blocks AMPA evoked depolarizations on motoneurones while responses to equi-effective doses of NMDA and DHPG were relatively unaffected. In the presence of 200 μM UBP-282, a concentration that completely abolishes AMPA-evoked depolarizations on motoneurones and kainate-evoked responses on dorsal root, there is still a noticeable depolarization evoked by kainate on motoneurones[1]. |
| In Vivo | On neonatal rat motoneurones UBP-282 (3-CBW) (200 μM) almost completely abolished responses to AMPA while responses to NMDA, kainate and DHPG were 101.6%, 39.4% and 110.5% of control, respectively. UBP-282 can therefore be used to isolate kainate receptor responses from those mediated by AMPA receptors[1]. |
| References |
| Molecular Formula | C15H15N3O6 |
|---|---|
| Molecular Weight | 333.29600 |
| Exact Mass | 333.09600 |
| PSA | 144.62000 |
|
~% 
544697-47-4 |
| Literature: Dolman, Nigel P.; Troop, Helen M.; More, Julia C. A.; Alt, Andrew; Knauss, Jody L.; Nistico, Robert; Jack, Samantha; Morley, Richard M.; Bortolotto, Zuner A.; Roberts, Peter J.; Bleakman, David; Collingridge, Graham L.; Jane, David E. Journal of Medicinal Chemistry, 2005 , vol. 48, # 24 p. 7867 - 7881 |
|
~% 
544697-47-4 |
| Literature: Dolman, Nigel P.; Troop, Helen M.; More, Julia C. A.; Alt, Andrew; Knauss, Jody L.; Nistico, Robert; Jack, Samantha; Morley, Richard M.; Bortolotto, Zuner A.; Roberts, Peter J.; Bleakman, David; Collingridge, Graham L.; Jane, David E. Journal of Medicinal Chemistry, 2005 , vol. 48, # 24 p. 7867 - 7881 |
|
~% 
544697-47-4 |
| Literature: Dolman, Nigel P.; Troop, Helen M.; More, Julia C. A.; Alt, Andrew; Knauss, Jody L.; Nistico, Robert; Jack, Samantha; Morley, Richard M.; Bortolotto, Zuner A.; Roberts, Peter J.; Bleakman, David; Collingridge, Graham L.; Jane, David E. Journal of Medicinal Chemistry, 2005 , vol. 48, # 24 p. 7867 - 7881 |
| Precursor 1 | |
|---|---|
| DownStream 0 | |