174636-32-9
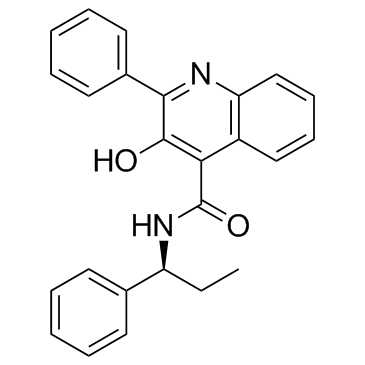
| Name | 3-hydroxy-2-phenyl-N-(1-phenylpropyl)quinoline-4-carboxamide |
|---|---|
| Synonyms |
Talnetant
(S)-N-(1-Phenylpropyl)-3-hydroxy-2-phenylquinoline-4-carboxamide,SB-223412 UNII-CZ3T9T146K |
| Description | Talnetant (SB 223412) is a potent and selective NK3 receptor antagonist (ki=1.4 nM, hNK-3-CHO); 100-fold selective for the hNK-3 versus hNK-2 receptor, with no affinity for the hNK-1 at concentrations up to 100 uM.IC50 Value: 1.4 nM (hNK-3-CHO binding Ki) [1]Target: NK3 receptorin vitro: In vitro studies demonstrated that 53 is a potent functional antagonist of the hNK-3 receptor (reversal of senktide-induced contractions in rabbit isolated iris sphincter muscles and reversal of NKB-induced Ca2+ mobilization in CHO cells stably expressing the hNK-3 receptor), while in vivo this compound showed oral and intravenous activity in NK-3 receptor-driven models (senktide-induced behavioral responses in mice and senktide-induced miosis in rabbits) [1]. Talnetant has high affinity for recombinant human NK3 receptors (pKi 8.7) and demonstrates selectivity over other neurokinin receptors (pKi NK2 = 6.6 and NK1<4). In native tissue-binding studies, talnetant displayed high affinity for the guinea pig NK3 receptor (pKi 8.5) [3].in vivo: Rectal barostat tests were performed on 102 healthy volunteers, randomized to receive either oral talnetant 25 or 100 mg or placebo over 14-17 days [2]. Talnetant (3-30 mg/kg i.p.) significantly attenuated senktide-induced 'wet dog shake' behaviors in the guinea pig in a dose-dependent manner. Microdialysis studies demonstrated that acute administration of talnetant (30 mg/kg i.p.) produced significant increases in extracellular dopamine and norepinephrine in the medial prefrontal cortex and attenuated haloperidol-induced increases in nucleus accumbens dopamine levels in the freely moving guinea pigs [3].Toxicity: Talnetant had no effect on rectal compliance, sensory thresholds or intensity ratings compared with placebo [2].Clinical trial: Study Of Talnetant Versus Placebo And Risperidone In Schizophrenia. Phase 2 |
|---|---|
| Related Catalog | |
| References |
| Density | 1.212g/cm3 |
|---|---|
| Boiling Point | 580.4ºC at 760mmHg |
| Molecular Formula | C25H22N2O2 |
| Molecular Weight | 382.45400 |
| Flash Point | 304.8ºC |
| Exact Mass | 382.16800 |
| PSA | 65.71000 |
| LogP | 6.06330 |
| Vapour Pressure | 4.51E-14mmHg at 25°C |
| Index of Refraction | 1.657 |
| Storage condition | 2-8℃ |
| Symbol |

GHS06 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H301 |
| Precautionary Statements | P301 + P310 |
| RIDADR | UN 2811 6.1 / PGIII |
| Precursor 5 | |
|---|---|
| DownStream 0 | |