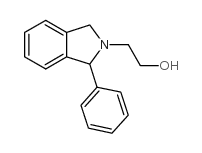
46868-19-3
| Name | 1-phenyl-3,4,5,6-tetrahydro-1H-2,5-benzoxazocine |
|---|---|
| Synonyms |
desmethyl nefopam
nor-nefopam 1H-2,5-Benzoxazocine,3,4,5,6-tetrahydro-1-phenyl |
| Description | N-Desmethylnefopam is the main metabolite of Nefopam. N-Desmethylnefopam is a centrally-acting but non-opioid analgesic agent, for the relief of moderate to severe pain. Nefopam targets β-catenin protein level in mesenchymal cells in-vitro and in-vivo[1][2][3]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.073g/cm3 |
|---|---|
| Boiling Point | 383.4ºC at 760 mmHg |
| Molecular Formula | C16H17NO |
| Molecular Weight | 239.31200 |
| Flash Point | 157.1ºC |
| Exact Mass | 239.13100 |
| PSA | 21.26000 |
| LogP | 3.22460 |
| Index of Refraction | 1.563 |
| Precursor 7 | |
|---|---|
| DownStream 2 | |