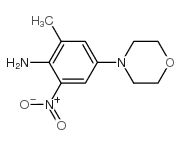
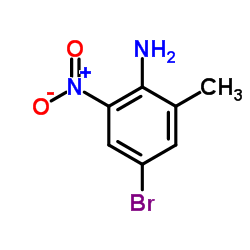
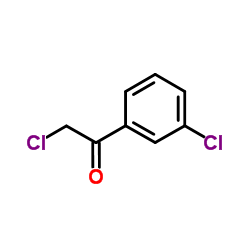
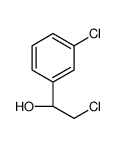
468740-43-4
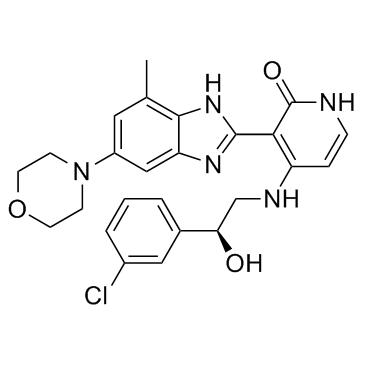
| Name | (S)-4-[2-(3-Chloro-phenyl)-2-hydroxy-ethylamino]-3-(4-methyl-6-morpholin-4-yl-1H-benzoimidazol-2-yl)-1H-pyridin-2-one |
|---|---|
| Synonyms |
CDK inhibitor
4-{[(2S)-2-(3-chlorophenyl)-2-hydroxyethyl]amino}-3-[4-methyl-6-(morpholin-4-yl)-1H-benzimidazol-2-yl]pyridin-2(1H)-one 4-{[(2S)-2-(3-Chlorophenyl)-2-hydroxyethyl]amino}-3-[4-methyl-6-(4-morpholinyl)-1H-benzimidazol-2-yl]-2(1H)-pyridinone CHEMBL401930 4-[[(2S)-2-(3-CHLOROPHENYL)-2-HYDROXYETHYL]AMINO]-3-[7-METHYL-5-(4-MORPHOLINYL)-1H-BENZIMIDAZOL-2-YL]-2(1H)-PYRIDINONE 2(1H)-Pyridinone, 4-[[(2S)-2-(3-chlorophenyl)-2-hydroxyethyl]amino]-3-[4-methyl-6-(4-morpholinyl)-1H-benzimidazol-2-yl]- INSULIN-LIKE GROWTH FACTOR-1 RECEPTOR INHIBITOR BMS-536924 |
| Description | BMS-536924 is an ATP-competitive IGF-1R/IR inhibitor with IC50 of 100 nM/73 nM.IC50 value: 100 nM (IGF-1R); 73 nM (IR) [1]Target: IGF-1R; IRin vitro: BMS-536924 also inhibits FAK and Lck with IC50 of 150 nM and 341 nM, respectively. BMS-536924 inhibits cellular proliferation and disrupts Akt and MAPK phosphorylation [1]. BMS-536924 inhibits IGF-I-stimulated IGF-1R signaling in MCF10A cells and blocks constitutive IGF-1R activity in CD8-IGF-1R-MCF10A. Preincubation of MCF10A cells with 1 μM BMS-536924 completely blocks the ability of IGF-I to stimulate IGF-1R phosphorylation. IGF-I stimulation results in increased phosphorylation of ERK1/2, GSK3β, and Akt. BMS-536924 inhibits this ligand-induced phosphorylation. Treatment of the CD8-IGF-1R-MCF10A cells with BMS-536924 results in a dose-dependent inhibition of phosphorylation with partial inhibition at 0.01 μM and 0.1 μM, but complete receptor inhibition at a concentration of 1 μM. Maximal inhibition of phosphorylated IGF-1R is observed as early as 10 minutes following incubation. BMS-536924 retains its ability to inhibit IGF-1R phosphorylation for up to 48 hours. Addition of BMS-536924 time-dependently inhibits Akt phosphorylation starting at 1 hour. By 48 hours, Akt activation is completely blocked [2]. in vivo: Oral administration of BMS-536924 at 100-300 mpk strongly inhibits IGR-1R Sal tumor model. Efficacy is also observed in the nonengineered Colo205 human colon carcinoma mode. Oral administration of 3 on a once a day schedule (100-300 mpk) or a twice a day schedule (50, 100 mpk) demonstrates antitumor activity in this tumor model. Oral glucose tolerance test (OGTT) shows 100 mpk (b.i.d.) causes a significant elevation in glucose levels after glucose challenge. The pharmacokinetic parameters of BMS-536924, administered orally in poly(ethylene glycol) 400 and water (80:20 v/v), are determined in mouse, rat, dog, and monkey. Good bioavailability is evident in all species. Significant nonlinear pharmacokinetics is observed in rodents at increasing p.o. dose [1]. Oral administration of 70 mg/kg BMS-536924 significantly inhibits tumor growth (TGBC-1TKB cells) inoculated in nude mice. BMS-536924 up regulates apoptosis in xenografts tumors. The treatment doesn't have adverse effects on the body weight of mice or the glucose levels at the time of death, suggesting tolerable toxicity [3]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Molecular Formula | C25H26ClN5O3 |
| Molecular Weight | 479.959 |
| Exact Mass | 479.172424 |
| PSA | 106.27000 |
| LogP | 2.61 |
| Index of Refraction | 1.717 |
| Storage condition | -20℃ |
|
~% 
468740-43-4 |
| Literature: Wittman, Mark; Carboni, Joan; Attar, Ricardo; Balasubramanian, Balu; Balimane, Praveen; Brassil, Patrick; Beaulieu, Francis; Chang, Chiehying; Clarke, Wendy; Dell, Janet; Eummer, Jeffrey; Frennesson, David; Gottardis, Marco; Greer, Ann; Hansel, Steven; Hurlburt, Warren; Jacobson, Bruce; Krishnananthan, Subramaniam; Lee, Francis Y.; Li, Aixin; Lin, Tai-An; Liu, Peiying; Ouellet, Carl; Sang, Xiaopeng; Saulnier, Mark G.; Stoffan, Karen; Sun, Yax; Velaparthi, Upender; Wong, Henry; Yang, Zheng; Zimmermann, Kurt; Zoeckler, Mary; Vyas, Dolatrai Journal of Medicinal Chemistry, 2005 , vol. 48, # 18 p. 5639 - 5643 |
|
~% 
468740-43-4 |
| Literature: Wittman, Mark; Carboni, Joan; Attar, Ricardo; Balasubramanian, Balu; Balimane, Praveen; Brassil, Patrick; Beaulieu, Francis; Chang, Chiehying; Clarke, Wendy; Dell, Janet; Eummer, Jeffrey; Frennesson, David; Gottardis, Marco; Greer, Ann; Hansel, Steven; Hurlburt, Warren; Jacobson, Bruce; Krishnananthan, Subramaniam; Lee, Francis Y.; Li, Aixin; Lin, Tai-An; Liu, Peiying; Ouellet, Carl; Sang, Xiaopeng; Saulnier, Mark G.; Stoffan, Karen; Sun, Yax; Velaparthi, Upender; Wong, Henry; Yang, Zheng; Zimmermann, Kurt; Zoeckler, Mary; Vyas, Dolatrai Journal of Medicinal Chemistry, 2005 , vol. 48, # 18 p. 5639 - 5643 |
|
~% 
468740-43-4 |
| Literature: Wittman, Mark; Carboni, Joan; Attar, Ricardo; Balasubramanian, Balu; Balimane, Praveen; Brassil, Patrick; Beaulieu, Francis; Chang, Chiehying; Clarke, Wendy; Dell, Janet; Eummer, Jeffrey; Frennesson, David; Gottardis, Marco; Greer, Ann; Hansel, Steven; Hurlburt, Warren; Jacobson, Bruce; Krishnananthan, Subramaniam; Lee, Francis Y.; Li, Aixin; Lin, Tai-An; Liu, Peiying; Ouellet, Carl; Sang, Xiaopeng; Saulnier, Mark G.; Stoffan, Karen; Sun, Yax; Velaparthi, Upender; Wong, Henry; Yang, Zheng; Zimmermann, Kurt; Zoeckler, Mary; Vyas, Dolatrai Journal of Medicinal Chemistry, 2005 , vol. 48, # 18 p. 5639 - 5643 |
|
~% 
468740-43-4 |
| Literature: Wittman, Mark; Carboni, Joan; Attar, Ricardo; Balasubramanian, Balu; Balimane, Praveen; Brassil, Patrick; Beaulieu, Francis; Chang, Chiehying; Clarke, Wendy; Dell, Janet; Eummer, Jeffrey; Frennesson, David; Gottardis, Marco; Greer, Ann; Hansel, Steven; Hurlburt, Warren; Jacobson, Bruce; Krishnananthan, Subramaniam; Lee, Francis Y.; Li, Aixin; Lin, Tai-An; Liu, Peiying; Ouellet, Carl; Sang, Xiaopeng; Saulnier, Mark G.; Stoffan, Karen; Sun, Yax; Velaparthi, Upender; Wong, Henry; Yang, Zheng; Zimmermann, Kurt; Zoeckler, Mary; Vyas, Dolatrai Journal of Medicinal Chemistry, 2005 , vol. 48, # 18 p. 5639 - 5643 |
| Precursor 4 | |
|---|---|
| DownStream 0 | |